Neurologists frequently encounter seizures related to stroke. Given that post-stroke epilepsy (PSE) is the most common form of acquired epilepsy, it is quite surprising that it has attracted little academic interest and that, so far, only scarce evidence is available to guide clinical practice. The scenario is, however, changing and both basic science researchers and clinical investigators have started to address highly relevant issues, including pathophysiology, prevalence and incidence, diagnosis, prevention, treatment, and prognosis.
When should PSE be diagnosed? When should treatment start? Which is the most effective treatment? Which antiepileptic drug (AED) should be preferred? Not all seizures occurring after stroke are necessarily stroke-related. So, when should PSE be diagnosed? Only an adequate knowledge and correct diagnosis may spare patients the anxiety, stigma, and side effects of unnecessary treatments.
In recent years, interesting developments in the field of epileptogenesis also suggests that the risk of PSE may be modified through pharmacological intervention. In addition, the association found between PSE and risk of vascular events highlights the importance of secondary stroke prophylaxis.
This seminar paper aims to provide an updated overview of PSE and offers clinical guidance to professionals involved in the treatment of patients with post-stroke seizures.
EARLY AND LATE POST-STROKE SEIZURES
DEFINITION
A distinction between early and late post-stroke seizures is mandatory in the field of seizures and stroke since it underscores different pathophysiological mechanisms. Early post-stroke seizures reflect an acute, and perhaps reversible cerebral injury (i.e. acute symptomatic, provoked) whereas late seizures arise from long-lasting changes in the post-stroke brain (i.e. remote symptomatic, unprovoked). In this article, we will use the terms “early and late post-stroke seizures” throughout. The distinction between early and late seizures is closely linked to the theoretical concept of epileptogenesis -the hereto incompletely characterized process by which the brain acquires an enduring predisposition to seizures. Epileptogenesis does not simply represent a process that starts at stroke onset and manifests with seizures at a later stage, but should be considered within the frame of a threshold model in which individual predisposition, stroke characteristics, and subsequent reactions to the primary injury converge with PSE.
The temporal limit to consider a seizure as a “late-seizure” ranges mostly between one and two weeks after stroke, in analogy with the concept of early and late post-traumatic seizures. A pivotal study in Rochester, Minnesota demonstrated that a seizure within seven days of a stroke carries a ten-year risk of a subsequent unprovoked seizure of 33% (95% confidence interval [CI]: 20.7-49.9), whereas a seizure occurring seven days after a stroke carries a ten-year risk of 71.5% (95% CI: 59.7-81.9) (Hesdorffer et al., 2009). Based on such differences in patient prognosis, seven days is currently the recommended cut-off for considering a post-stroke seizure as early or late (Beghi et al., 2010). According to the most recent diagnostic criteria, epilepsy can be diagnosed after a single seizure and with a recurrence risk >60% within the next ten years (Fisher et al., 2014). As shown by the Rochester study, patients presenting with a single late seizure after a stroke carry such a risk. Therefore, one late unprovoked post-stroke seizure can be diagnosed as PSE.
However, it is important to recognize the pitfalls of the seven-day cut-off in order to distinguish between early and late seizures (see below under: “When and how to clinically diagnose post-stroke epilepsy”). The risk of PSE is substantially higher in patients who have presented with an early post-stroke seizure than in patients who have not had any seizure. The occurrence of early seizures is, indeed, an independent risk factor for PSE and weighs heavily in the SeLECT score. The SeLECT score is a recently developed and validated clinical tool to predict late seizures/epilepsy after ischaemic stroke. In addition to the occurrence of an early seizure, it takes into account the severity of stroke, aetiology of stroke, and cortical and arterial territory involved (Ferlazzo et al., 2016; Galovic et al., 2018). An early seizure, not considered epilepsy, should therefore not convey the message that the patient is at no risk of PSE. Clinical risk models and biomarkers must be incorporated in the future to help identify the mechanisms of PSE and refine the diagnosis of PSE in some patients with early seizures and reassure those at low risk of recurrence.
PATHOPHYSIOLOGY
Much of the pathophysiology underlying seizures after stroke remains elusive. Experimental stroke studies have mapped a number of reactions following brain injury, which are common to other models of acquired epilepsy and include inflammatory response, changes in the expression of proteins involved in neuronal signalling, and remodelling of cytoskeleton, but causal links have not been clearly established. Increased blood-brain barrier permeability could also play a pathogenic role (Pitkänen et al., 2016).
Early post-stroke seizures should be regarded as a reaction of the neuronal cells to the acute cerebrovascular injury. They reflect transient cellular biochemical dysfunctions, including -among others- the increased release of excitatory neurotransmitter glutamate, ionic imbalance, breakdown of membrane phospholipids, and release of free fatty acids with oxidative stress (Tanaka and Ihara, 2017). Homeostatic or systemic disturbances, such as electrolyte imbalance, acid-base disturbances and hyperglycaemia, may also play a role in the development of early post-stroke seizures (Tanaka and Ihara, 2017). Conversely, late post-stroke seizures reflect a structural change of neuronal networks following the cerebrovascular injury to the brain (Trinka and Brigo, 2014). They are usually attributed to epileptogenic gliotic scarring with changes in membrane properties, neuronal deafferentation, selective neuronal loss or collateral sprouting (Tanaka and Ihara, 2017). Late post-stroke seizures after a primary cerebral haemorrhage or secondary haemorrhagic transformation of an ischemic stroke are thought to be the consequence of haemosiderin deposits leading to increased neuronal excitability. During post-stroke epileptogenesis, the brain undergoes molecular and cellular alterations, which increase its excitability and eventually lead to the occurrence of recurrent spontaneous seizures. These progressive neuronal changes include selective neuronal cell death and apoptosis, changes in membrane properties, mitochondrial and receptor changes (e.g. loss of GABAergic receptors), deafferentation, and collateral sprouting (Pitkänen et al., 2016). Disruption of the brain-blood barrier following endothelial damage causes extravasation of albumin which in turns activates astrocytes and microglial cells; this leads to changes in the extracellular milieu with increased glutamate levels, release of inflammatory cytokines, and further increase in brain-blood barrier permeability (Tanaka and Ihara, 2017). Thrombin, a major component of the coagulation cascade, and its protease-activated receptor 1 (PAR1), may further contribute to maladaptive plasticity leading to permanent structural changes in the brain with altered neuronal firing and circuit dysfunctions (Altman et al., 2019). This complex cascade of events directly enhances neuronal excitability and could explain epileptogenesis after a stroke. Alterations in gene expression after a stroke can also play a role in epileptogenesis, as they can be associated with impaired neuroprotection, aberrant synaptic plasticity, upregulation of neuronal excitability, and enhanced gliotic scarring formation (Pitkänen et al., 2016). Of note, these pathophysiological mechanisms interact with each other and eventually lead to structural and functional alterations of neuronal networks, leading to recurrent spontaneous seizures (Tanaka and Ihara, 2017).
Remarkably, the current pathophysiological perspective of acquired epilepsy favours a threshold model, which also involves individual predisposition. For instance, individuals with a first-degree relative suffering with epilepsy are at higher risk of developing PSE (hazard ratio: 1.18; 95% CI: 1.09-1.28) although this was associated with a small effect size (Eriksson et al., 2019). Lesion characteristics may be more important in most cases such as size of the lesion, cortical involvement and presence of intralesional blood products (see below under “Neuroimaging of post-stroke seizures: pitfalls and differential diagnosis”).
Until now, the early treatment of stroke patients with AEDs during the acute phase has not been effective in reducing the risk of developing PSE (Gilad et al., 2011; Sheth et al., 2015). On the other hand, statins appear to be the only medication to decrease the risk of PSE (Etminan et al., 2010), and to a greater extent in patients who present with early seizures and are considered a high-risk group (Guo et al., 2015). However, causality and mechanisms of the effect of statins are not yet well-established.
EPIDEMIOLOGY AND RISK FACTORS
The rates of early post-stroke seizures and PSE vary across stroke populations. For ischaemic stroke, the prevalence of early seizures is generally 3-6% (Beghi et al., 2011; Labovitz et al., 2001; Guo et al., 2015; Serafini et al., 2015) but can be up to 15% in selected cohorts (Labovitz et al., 2001; Lamy et al., 2003; Bentes et al., 2017). There is no converging evidence about the risk of early seizures in patients treated with reperfusion therapies, either intravenous thrombolysis or endovascular thrombectomy (Belcastro et al., 2020; Brigo et al., 2020a; Feher et al., 2019). The risk of intracerebral haemorrhage is somewhat higher (Qian et al., 2014), with early seizures occurring in approximately 10-16% of patients (Naess et al., 2004; Beghi et al., 2011; Procaccianti et al., 2012). However, the methodology adopted to ascertain and diagnose early post-stroke seizures can greatly affect the results. For instance, a study using video-EEG recording performed in the first 72 hours following an acute anterior circulation ischaemic stroke revealed early seizures in 14.6% and non-convulsive status epilepticus (SE) in 2.6% of patients; of note, almost a quarter (22.7%) of early seizures were exclusively electrographic (Bentes et al., 2017).
Data on PSE prevalence also depend on the study population and methodology used to collect data. Based on nationwide registers in Sweden, the cumulative incidence of PSE was 6.4% following ischaemic stroke and 12.4% following haemorrhagic stroke after a follow-up of almost five years (Zelano et al., 2016); the latter finding has been replicated in a population-based investigation in a Finnish region (Lahti et al., 2017). In a video-EEG study, 15.2% of patients suffering with an anterior ischemic stroke met the diagnostic criteria for epilepsy at 12 months (Bentes et al., 2017).
A diagnosis of PSE (after ischaemic and haemorrhagic stroke) increases the risk of mortality after adjusting for stroke severity (Zelano et al., 2016) and, unsurprisingly, vascular disease is the major cause of death. These findings call for concerted efforts to prioritise and optimize secondary vascular prophylaxis (Hansen et al., 2017), and AEDs that do not interfere with concomitant medications, such as anti-hypertensives and anticoagulants, should be preferentially chosen.
The main risk factors for early post-ischaemic stroke seizures are cortical involvement, severe stroke, haemorrhagic transformation, age younger than 65 years, a large lesion and atrial fibrillation (Feher et al., 2019). The main risk factors for PSE following ischaemic stroke are cortical involvement, haemorrhage, and early seizures (Ferlazzo et al., 2016).
WHEN AND HOW TO CLINICALLY DIAGNOSE POST-STROKE EPILEPSY
The concept of early and late seizures and PSE is straightforward to apply in clinical practice in most cases. If a patient has a seizure within a week of stroke, it is an early seizure and considered acute symptomatic. Although such a seizure carries a risk of subsequent epilepsy, this risk does not warrant the diagnosis of PSE. In contrast, a seizure occurring more than one week after stroke is considered an unprovoked late seizure. This infers a >60% risk of seizure recurrence and the patient meets the diagnostic criteria for epilepsy.
In some circumstances, the distinction between early and late seizures may not be unequivocal. The clinical situation may have been unstable, and the exact time of the latest cerebral insult may not be clear. As per the definition of epilepsy recommended by the International League Against Epilepsy, the diagnosis requires a risk of seizure recurrence exceeding 60%, however, the exact risk in each case is hard to estimate with precision. If there is doubt whether a seizure has occurred within the acute symptomatic phase, then there is no clear evidence of a >60% recurrence risk. In this scenario, the diagnosis of PSE should not be made. A similar approach can be suggested if there is doubt whether a paroxysmal post-stroke event is actually a seizure. In the presence of uncertainty, it is probably better not to diagnose a late seizure/PSE, but rather adopt a wait-and-watch approach. It is important to emphasize, however, that whether a patient is or is not diagnosed with PSE, the decision to initiate treatment with AEDs will depend on clinical characteristics of individual patients.
THE ROLE OF THE ELECTROENCEPHALOGRAM IN THE DIAGNOSIS AND PREDICTION OF POST-STROKE SEIZURES
In the early phase following an ischaemic or haemorrhagic stroke, electroencephalogram (EEG) is an essential diagnostic tool that aims to detect purely electrographic seizures. It can also detect specific patterns, such as lateralized periodic discharges (LPDs), that are independently associated with early seizures (Mecarelli et al., 2011).
Interestingly, brain single-photon emission computed tomography (SPECT) imaging can reveal focal hypermetabolism with increased cerebral blood flow in association with LPDs in patients with post-stroke seizures; such findings support the view that – at least in some patients – this EEG pattern may correspond to an ictal phenomenon (Ergün et al., 2006; Hughes, 2010).
The lack of a systematic electrophysiological assessment with video-EEG can lead to an underestimation of seizures, particularly in the case of focal unaware or non-convulsive seizures (Belcastro et al., 2014; Bentes et al., 2017; Brigo et al., 2020a, 2020b). Neurologists and health personnel working in stroke units should promptly request an EEG recording for patients with sudden onset of unexplained behavioural changes or impairment of consciousness. A continuous EEG lasting ≥24 hours should be recorded as soon as possible in patients with acute supratentorial brain injury presenting with altered mental status or with clinical paroxysmal events suspected to be seizures. In addition, in comatose patients, patients with periodic discharges, or patients who are pharmacologically sedated, a more prolonged EEG (≥48 hours) may lead to the detection of non-convulsive seizures (Herman et al., 2015). The main indications for continuous EEG in patients with acute stroke, to identify non-convulsive seizures and non-convulsive status epilepticus, are presented in table 1.
EEG recordings may also have implications in the prediction of functional outcome, mortality and post-stroke cognitive decline, with different levels of evidence (Doerrfuss et al., 2020).
Only few studies have, so far, assessed EEG as a predictive tool for post-stroke seizures and epilepsy. Abnormalities on EEG can predict the development of epilepsy in the first year after stroke, independently of clinical and imaging-based infarct severity. A retrospective study of 110 patients with ischaemic stroke-related seizures found LPDs in 5.8% of patients, whereas the 275 stroke patients who did not suffer an early and/or a late seizure did not present with LPDs (De Reuck et al., 2006). Diffuse EEG slowing and frontal intermittent rhythmic delta activities also occurred more frequently among patients with post-stroke seizures compared to controls (21.7% versus 5.1% and 24.6% versus 1.1%, respectively) (De Reuck et al., 2006). A prospective video-EEG study enrolled 151 patients with anterior circulation ischaemic stroke and no previous seizures. Asymmetric background activity and interictal epileptiform activity detected on EEG performed during the first 72 hours after stroke were independent predictors of PSE during the first year following the index event (Bentes et al., 2018).
These findings suggest how EEG recorded in the acute stroke phase may not only detect subclinical seizures, but also may provide useful information to predict the development of PSE. Further studies are warranted to assess whether the inclusion of EEG findings with existing scores (e.g. SeLECT [Galovic et al., 2018]) could improve their predictive accuracy (Doerrfuss et al., 2020).
Most studies available in the literature refer to the use of EEG in the acute phase as a predictor of unprovoked late post-stroke seizures. In patients with PSE, EEG usually shows multifocal or focal slowing, typically with a normal background alpha rhythm (Mecarelli and Vicenzini, 2019). Epileptiform abnormalities can be detected, usually as sharp waves, sometimes with a quasiperiodic pattern of recurrence, particularly in PSE associated with large cortical cerebrovascular lesions (Brigo and Mecarelli, 2019; Mecarelli and Vicenzini, 2019).
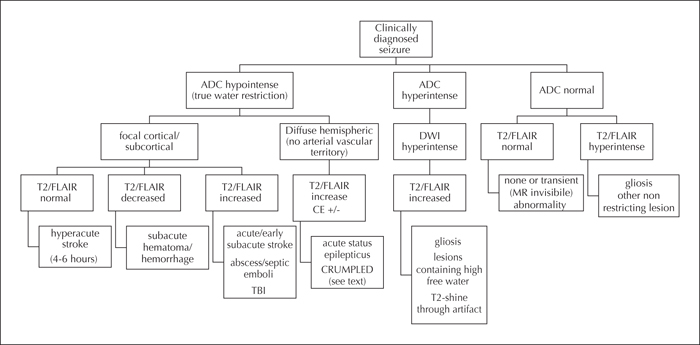
NEUROIMAGING OF POST-STROKE SEIZURES: PITFALLS AND DIFFERENTIAL DIAGNOSIS
Seizures are an expression of sudden depolarization of neurons that transiently disrupts ionic and metabolic homeostasis. There are different proposed pathophysiological mechanisms for early and late seizures, which include critically reduced local blood flow, abnormal release of neurotransmitters, metabolic dysfunction, presence of gliotic scarring and aberrant synaptic connectivity (Pitkänen et al., 2016). Of note, only a minority of stroke patients will develop seizures and there is still scarce understanding of magnetic resonance imaging (MRI) signatures that can identify the patients at higher risk. Some studies have identified the following MRI predictors: watershed infarctions, middle cerebral arterial territory strokes, cortical involvement, haemorrhagic strokes and haemorrhagic transformation of ischaemic stroke (Ferlazzo et al., 2016; Galovic et al., 2018).
Caution is, however, necessary in considering a post-stroke seizure as stroke related. In such cases, neuroimaging is fundamental in providing a differential diagnosis.
In acute settings, cranial computed tomography (CCT) is the gold standard to rapidly image patients presenting with seizures. It also represents the only available neuroimaging tool in patients who cannot undergo MRI. Besides standard CCT, perfusion CT (PCT) can be helpful in differentiating between stroke, stroke mimics and status epilepticus (Strambo et al., 2018). Ongoing seizure activity or SE are characterized by regions of hyperperfusion that usually involve atypical vascular territories, whereas strokes typically correspond to hypoperfused areas in a precise arterial territory (Payabvash et al., 2015). PCT can also be helpful to differentiate postictal versus stroke related focal neurological deficits: the former are characterized by transient iso- to hyperperfusion and the latter by areas of hypoperfusion in a vascular territory (Brigo and Lattanzi, 2020). Notably, PCT must be performed within a strict interval from seizure onset (< three hours) to improve its sensitivity (Payabvash et al., 2015).
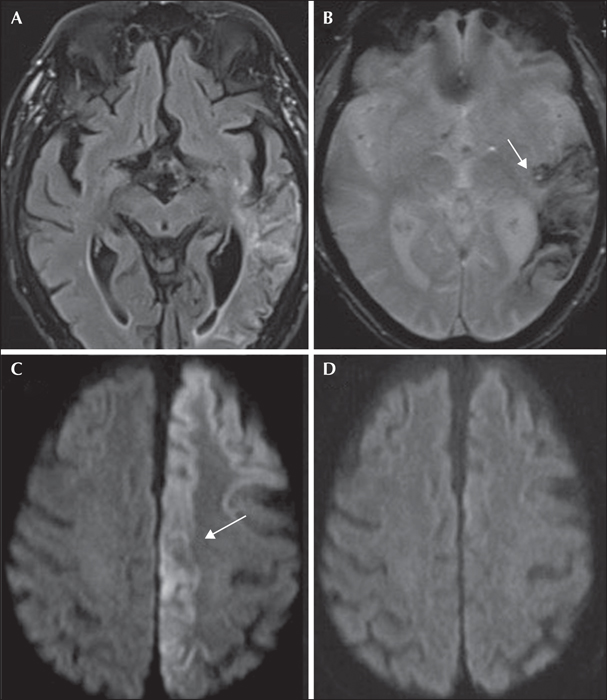
MRI remains the most sensitive non-invasive diagnostic tool to image the brain, and conventional MR sequences suffice in most cases of suspected post-stroke seizures. The most common conventional sequences are listed in table 2. Diffusion-weighted imaging (DWI) is an informative sequence and is now routinely used in clinical settings. It is fast (acquisition requires less than a minute) and demonstrates high sensitivity for areas of water restriction, making it a very commonly used sequence to differentiate stroke from stroke mimics. Nonetheless, timing of acquisition is extremely important to avoid pitfalls. Lesions can be falsely positive (those containing a very high water content, also known as “T2 shine-through” artefact) or falsely negatively (MRI scans acquired too late after symptom onset) (Agarwal et al., 2017; Shono et al., 2017). Restricted signal on DWI due to seizure activity is mostly reversible (figure 1), whereas it may last three to four days in stroke, unless cerebral tissue has been reperfused earlier. Other lesions that may present with DWI positive signal include subacute haematomas, hypercellular tumours and abscesses (figure 2). Recently, Koksel et al. proposed the acronym “CRUMPLED” as a helpful way to remember the most important DWI-restricted lesions that are cortically based and have an atypical vascular distribution. The acronym stands for C = Creutzfeldt-Jakob disease; R = reversible cerebral vasoconstriction syndrome; U = urea cycle disorders and uraemia; M = mitochondrial disorders; P = prolonged seizures and posterior reversible encephalopathy syndrome (PRES); L = laminar necrosis (hypoxic-ischaemic encephalopathy) and liver-related (acute hepatic or hyperammonaemic encephalopathy); E=encephalitis (infectious meningoencephalitis) and D = diabetes mellitus (hypoglycaemia) (Koksel et al., 2018) (figure 2).
Intravenous gadolinium-based imaging can be particularly useful to: a) identify areas of disrupted blood-brain barrier; b) provide evidence of reperfusion or presence of collateral flow; and c) identify stroke mimics.
Advanced imaging can provide additional and more accurate information for the differential diagnosis.
Perfusion weighted imaging (PWI) can reliably identify tissue at risk of infarct, defined as an area with a blood flow of less than 50 mL per 100 mL of brain tissue per minute (Jahng et al., 2014). Signal changes in PWI are related to electrographic ictal activity: hyperperfusion is likely to be seen in pre-ictal and ictal phases, whereas hypoperfusion is more common in the post-ictal phase (Takahara et al., 2018). Early seizures are more likely to present as areas of hyperperfusion due to the underlying pathophysiological mechanisms, including metabolic dysfunction and abnormal release of neurotransmitters, whereas late seizures are likely to show a mixed pattern of perfusion as they are more related to gliotic changes and loss of neuronal tissue (Yoo et al., 2017). Hyperperfusion may also precede DWI signal changes as a compensatory mechanism to support the abnormally increased depolarization of neurons (Takahara et al., 2018). Susceptibility weighted imaging (SWI) and gradient-echo (GRE) sequences can be highly informative and detect punctuate microbleeds and areas of iron-laden products in patients with subarachnoid haemorrhage, chronic subdural haematoma, cerebral amyloid angiopathy or superficial siderosis. Extracellular haemosiderin is considered epileptogenic and may cause focal cerebral irritation and initiate seizures, even though the mechanisms are not yet well-established (O’Connor et al., 2014).
MANAGEMENT OF ACUTE-SYMPTOMATIC POST-STROKE SEIZURES
Due to the rather low risk of early, acute-symptomatic post-stroke seizures, ranging from 3-6% in cases of cerebral ischaemia to 16% in primary cerebral haemorrhage (Labovitz et al., 2001; Naess et al., 2004; Beghi et al., 2011; Procaccianti et al., 2012; Guo et al., 2015; Serafini et al., 2015), primary prophylaxis with an AED is not recommended. This is also true for those patients who have cerebral haemorrhage involving cortical structures and a risk of early post-stroke seizures of around 35%. If physicians decide to introduce primary AED prophylaxis despite the evidence-based recommendations, an AED that can be titrated very quickly, administered intravenously, and which lacks significant drug-drug interactions should be preferred. One of the most commonly prescribed AEDs that meets these characteristics is levetiracetam (LEV). One randomized controlled trial compared valproate to placebo in 36 patients, both of which were administered directly after intracerebral haemorrhage (Gilad et al., 2011). The groups did not differ with respect to prevention of early post-stroke seizures (defined in that study as occurring within the first 14 days), but the trial was underpowered, and prevention of early seizures was not the primary endpoint.
After the occurrence of one early post-stroke seizure, the risk of developing a second acute symptomatic seizure within the acute phase is only 10-20% (De Herdt et al., 2011; Leung et al., 2017). Due to the low risk of recurrence, guidelines generally do not recommend secondary AED prophylaxis after an early post-stroke seizure (Holtkamp et al., 2017). However, many clinicians prefer to administer an AED to reduce the likelihood of clinical worsening in the acute setting. Conceptually, this approach likely relies on pathophysiological considerations including increased neuronal excitotoxicity, peri-infarct depolarisations, and inflammatory responses in the first hours and days after stroke (Dirnagl et al., 1999), all of which can be risk factors for acute recurrence of epileptic seizures. The criteria used to choose the AED for acute secondary prophylaxis are similar to those for primary prophylaxis.
If patients without or after an early post-stroke seizure have been administered an AED, physicians are encouraged to withdraw it after the acute phase – at best, at discharge from the stroke unit – as the vast majority of these patients will not experience any future seizures (Holtkamp et al., 2017). The risk of a first unprovoked post-stroke seizure within eight years (which would define epilepsy) after cerebral infarct is 8% and 15% after cerebral haemorrhage (Merkler et al., 2018), and the risk of an unprovoked seizure after one early post-stroke seizure with 10 years is 30 to 35% (Hesdorffer et al., 2009; Galovic et al., 2018). Two studies developed scores to estimate the long-term risk of unprovoked seizures after acute cerebrovascular events. The CAVE score indicates a five-year seizure risk of 46.2% in patients after intracerebral haemorrhage based on the following four variables: early post-stroke seizure(s), cortical involvement, bleeding volume of more than 10 mL, and age of less than 65 years (Haapaniemi et al., 2014). The SeLECT score indicates a five-year seizure risk of more than 50% in patients after ischaemic stroke based on the following four or five criteria: early post-stroke seizure(s), severe stroke (NIHSS ≥11), cortical involvement, and large-artery atherosclerosis and/or involvement of the middle cerebral artery territory (Galovic et al., 2018). In these individual risk constellations, long-term secondary AED prophylaxis may be indicated.
MANAGEMENT OF POST-STROKE EPILEPSY
AED treatment is advised based on guidelines when PSE is diagnosed (Holtkamp et al., 2017). As always, there may be individual reasons not to start treatment -for instance, in cases with very mild semiology. Regarding the selection of drugs, two underpowered randomized, open-label studies compared controlled-release carbamazepine (CBZ-CR) to lamotrigine (LTG) (Gilad et al., 2007) and LEV (Consoli et al., 2012). The 12-month seizure freedom rates were 44% and 85% for CBZ-CR and 72% and 94% for LTG and LEV, without significant differences. LTG and LEV were better tolerated than CBZ-CR. A network meta-analysis of these trials showed no difference between LEV and LTG for seizure freedom (OR: 0.86; 95% CI: 0.15-4.89), but demonstrated greater occurrence of adverse events for LEV than LTG (OR: 6.87; 95% CI: 1.15-41.1) (Brigo et al., 2018). A randomized double-blinded trial on AEDs in epileptic patients, aged 60 years and older (two thirds had cerebrovascular aetiology), demonstrated higher one-year retention rates for LEV (62%) compared to CBZ-CR (46%; p=0.02), while LTG (56%) was intermediate (Werhahn et al., 2015). The SANAD trial, a non-blinded randomized controlled study comparing five standard and new AEDs in focal epilepsy, found LTG to have the best retention rate as compared to carbamazepine (CBZ), gabapentin, oxcarbazepine, and topiramate (Marson et al., 2007). Although data were not stratified according to the underlying aetiology, the findings can likely be extrapolated to PSE. The non-blinded, randomized, 52-week KOMET study compared the effectiveness of LEV as monotherapy to extended-release sodium valproate (VPA-ER) or CBZ-CR after the physician had decided which of the two AEDs best suited the individual patient (Trinka et al., 2013). In a post-hoc subgroup analysis of patients aged ≥60 years with newly diagnosed epilepsy (most of which were likely to have cerebrovascular aetiology), the 12-month retention rates in the VPA-ER stratum were 90% in the LEV group and 77% in the VPA-ER group; the corresponding rates in the CBZ-CR stratum were 75% and 53% in the LEV and CBZ-CR treatment arms, respectively (Pohlmann-Eden et al., 2016). In summary, the findings from clinical studies argue in favour of the newer AEDs for PSE due to their better tolerability profiles.
In focal epilepsy, the underlying aetiology does not usually determine the choice of AED. The decision regarding the most suitable compound has to be individualized according to the patient’s age, sex, comorbidities and comedications. Patients with PSE likely carry some burden of cardiovascular risk factors. Accordingly, AEDs such as CBZ, phenytoin, phenobarbital and primidone, which can increase biochemical markers of vascular disease, including total cholesterol, lipoprotein, C-reactive protein and homocysteine (Mintzer et al., 2009), should be avoided. Being strong enzyme-inducers, these AEDs may also increase the metabolism, and thus decrease serum concentrations, of drugs that are concomitantly administered for stroke management, such as warfarin. Post-stroke depression is common, and the detrimental effects of LEV on behaviour (Josephson et al., 2019) may further fuel psychiatric comorbidity, rendering this AED less appropriate in patients with post-stroke depression.
The question to withdrawal the antiepileptic treatment at some time point after the onset of PSE is difficult to address. The overall risk of seizure recurrence within five years after AED tapering is roughly 50%. A meta-analysis on seizure recurrence rate after AED withdrawal, based on 10 retrospective, prospective and randomized-controlled trials involving more than 1,700 patients, allowed the development of a prediction tool for seizure relapse (Lamberink et al., 2017). This tool can be accessed online (Epilepsy Prediction and Tool., 2019) and can assist physicians, but the decision to withdraw the treatment needs to be tailored to each patient individually.
FUTURE PERSPECTIVES
Several issues of PSE remain open to further research and investigation. Studies are warranted to elucidate the mechanisms of epileptogenesis after stroke and identify reliable biomarkers associated with the development of PSE. The role of EEG in predicting the occurrence of post-stroke seizures and epilepsy requires additional evaluation. The duration of EEG recording should be further evaluated in order to establish whether prolonged video-EEG monitoring during the first 72 hours after stroke is cost-effective and can offer advantages over routine, short-lasting EEG to identify post-stroke seizures (Grillo, 2015). The association of systemic thrombolysis and mechanical revascularization procedures with the development of early and late post-stroke seizures is still a matter of debate (Bentes et al., 2020). Similarly, there remain uncertainties about the most efficacious and safe AED to manage PSE.
Long-term, prospective, multicentric, high-quality studies with large cohorts of patients and stroke registries are needed to elaborate a practice guideline on diagnosis and treatment of PSE.
SUPPLEMENTARY DATA
Summary didactic slides are available on the www.epilepticdisorders.com website.
Illustrations
Tableaux
DISCLOSURES
Dr. Brigo received travel support from Eisai; acted as consultant for Eisai, LivaNova, and UCB Pharma; and was one of the organizers of the “Seizures & Stroke” Congress, held in Gothenburg from 20th to 22nd February 2019.
Dr Zelano has received consultancy fees from the Swedish Medial Product agency; speaker honoraria from UCB, was one of the organizers of the “Seizures & Stroke” Congress, held in Gothenburg from 20th to 22nd February 2019; and as an employee of Sahlgrenska university hospital (no personal compensation) is, and has been, an investigator in clinical trials sponsored by GW Pharma, SK life science, UCB, and Bial.
Dr. Holtkamp received speaker’s honoraria and/or consultancy fees from Bial, Desitin, Eisai, GW Pharmaceuticals, LivaNova, Novartis, and UCB (within the last three years).
Dr. Trinka received speaker honoraria from Eisai, UCB Pharma, LivaNova, Sandoz, Novartis, Biogen, Everpharma, BIAL-Portela &C, Newbridge, GL Pharma, Boehringer; grants from Biogen, UCB Pharma, Bayer, Novarti, Eisai, Merck, and Red Bull; grants from the European Union, FWF Österreichischer Fond zur Wissenschaftsforderung, Bundesministerium für Wissenschaft und Forschung, and Jubiläumsfond der Österreichischen Nationalbank outside the submitted work; and is a member of the following ILAE Task forces: Medical Therapies, Nosology, Terminology, Congresses, Driving, Regulatory affairs, and Telemedicine.
Dr. Agarwal and Dr. Lattanzi have no conflicts of interest to disclose.