ABSTRACT
Interactive brain stimulation is a new generation of neurofeedback characterized by a radical change in the targets of cognitive (volitional, adaptive) influence. These targets are represented by specific cerebral structures and neural networks, the reconstruction of which leads to the brain functions’ restoration and behavioral metamorphoses.
Functional magnetic resonance imaging (fMRI) in the neurofeedback contour uses a natural intravascular tracer, a blood-oxygenation-level-dependent (BOLD) signal as feedback. The subject included into the “interactive brain contour” learns to modulate and modify his or her own cerebral networks, creating new ones or “awakening” pre-existing ones, in order to improve (or restore) mental, sensory, or motor functions.
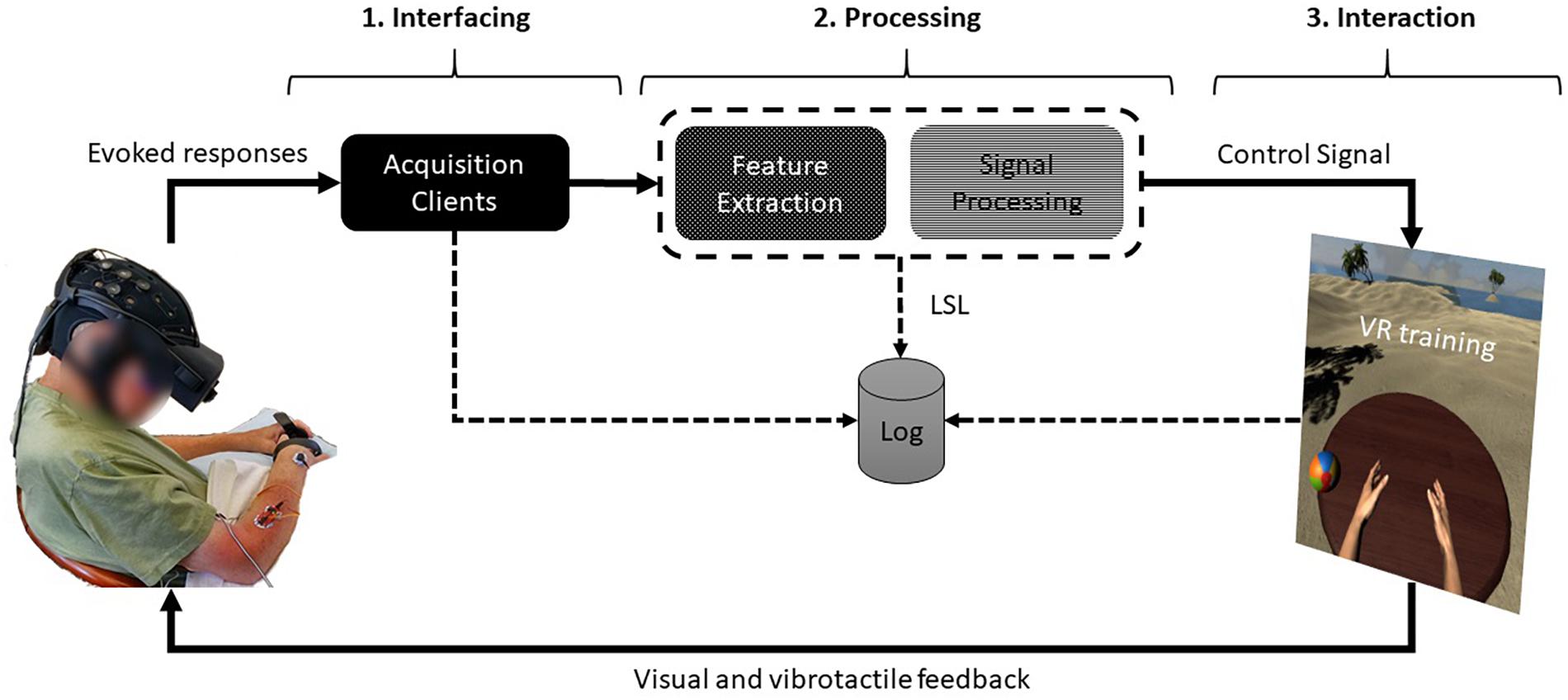
In this review we focus on interactive brain stimulation based on BOLD signal and its role in the motor rehabilitation of stroke, briefly introducing the basic concepts of the so-called “network vocabulary” and general biophysical basis of the BOLD signal. We also discuss a bimodal fMRI-EEG neurofeedback platform and the prospects of fMRI technology in controlling functional connectivity, a numerical assessment of neuroplasticity.
REFERENCES
Alionte, C., Notte, C., & Strubakos, C. D. (2022). From symmetry to chaos and back: Understanding and imaging the mechanisms of neural repair after stroke. Life Sciences, 288, Article 120161. https://doi.org/10.1016/j.lfs.2021.120161
Almeida, S. R. M., Vicentini, J., Bonilha, L., De Campos, B. M., Casseb, R. F., & Min, L. L. (2017). Brain connectivity and functional recovery in patients with ischemic stroke. Journal of Neuroimaging, 27(1), 65–70. https://doi.org/10.1111/jon.12362
Alstott, J., Breakspear, M., Hagmann, P., Cammoun, L., & Sporns, O. (2009). Modeling the impact of lesions in the human brain. PLoS Computational Biology, 5(6), Article e1000408. https://doi.org/10.1371/journal.pcbi.1000408
Alves, R., Henriques, R. N., Kerkelä, L., Chavarrías, C., Jespersen, S. N., & Shemesh, N. (2022). Correlation tensor MRI deciphers underlying kurtosis sources in stroke. NeuroImage, 247, Article 118833. https://doi.org/10.1016/j.neuroimage.2021.118833
Bassett, D. S., Meyer-Lindenberg, A., Achard, S., Duke, T., & Bullmore, E. (2006). Adaptive reconfiguration of fractal small-world human brain functional networks. Proceedings of the National Academy of Sciences of the United States of America, 103(51), 19518–19523. https://doi.org/10.1073/pnas.0606005103
Bentley, W. J., Li, J. M., Snyder, A. Z., Raichle, M. E., & Snyder, L. H. (2016). Oxygen level and LFP in task-positive and task-negative areas: Bridging BOLD fMRI and electrophysiology. Cerebral Cortex, 26(1), 346–357. https://doi.org/10.1093/cercor/bhu260
Bezmaternykh, D. D., Kalgin, K. V., Maximova, P. E., Mel’nikov, M. Y., Petrovskii, E. D., Predtechenskaya, E. V., Savelov, A. A., Semenikhina, A. A., Tsaplina, T. N., Shtark, M. B., & Shurunova, A. V. (2021). Application of fMRI and simultaneous fMRI-EEG neurofeedback in post-stroke motor rehabilitation. Bulletin of Experimental Biology and Medicine, 171(3), 379–383. https://doi.org/10.1007/s10517-021-05232-1
Bezmaternykh, D. D., Mel’nikov, M. E., Petrovskii, E. D., Kozlova, L. I., Shtark, M. B., Savelov, A. A., Shubina, O. S., & Natarova, K. A. (2018). Spontaneous changes in functional connectivity of independent components of fMRI signal in healthy volunteers at rest and in subjects with mild depression. Bulletin of Experimental Biology and Medicine, 165(3), 325–330. https://doi.org/10.1007/s10517-018-4161-3
Birn, R. M., Bandettini, P. A., Cox, R. W., & Shaker, R. (1999). Event-related fMRI of tasks involving brief motion. Human Brain Mapping, 7(2), 106–114. https://doi.org/10.1002/(SICI)1097-0193(1999)7:2<106::AID-HBM4>3.0.CO;2-O
Biswal, B., Yetkin, F. Z., Haughton, V. M., & Hyde, J. S. (1995). Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magnetic Resonance in Medicine, 34(4), 537–541. https://doi.org/10.1002/mrm.1910340409
Bonmassar, G., Anami, K., Ives, J., & Belliveau, J. W. (1999). Visual evoked potential (VEP) measured by simultaneous 64-channel EEG and 3T fMRI. NeuroReport, 10(9), 1893–1897. https://doi.org/10.1097/00001756-199906230-00018
Buckner, R. L. (1998). Event-related fMRI and the hemodynamic response. Human Brain Mapping, 6(5–6), 373–377 https://doi.org/10.1002/(SICI)1097-0193(1998)6:5/6<373::AID-HBM8>3.0.CO;2-P
Buckner, R. L., & DiNicola, L. M. (2019). The brain’s default network: Updated anatomy, physiology and evolving insights. Nature Reviews Neuroscience, 20(10), 593–608. https://doi.org/10.1038/s41583-019-0212-7
Carter, A. R., Astafiev, S. V., Lang, C. E., Connor, L. T., Rengachary, J., Strube, M. J., Pope, D. L. W., Shulman, G. L., & Corbetta, M. (2010). Resting interhemispheric functional magnetic resonance imaging connectivity predicts performance after stroke. Annals of Neurology, 67(3), 365–375. https://doi.org/10.1002/ana.21905
Carter, A. R., Shulman, G. L., & Corbetta, M. (2012). Why use a connectivity-based approach to study stroke and recovery of function? NeuroImage, 62(4), 2271–2280. https://doi.org/10.1016/j.neuroimage.2012.02.070
Cheng, H.-J., Ng, K. K., Qian, X., Ji, F., Lu, Z. K., Teo, W. P., Hong, X., Nasrallah, F. A., Ang, K. K., Chuang, K.-H., Guan, C., Yu, H., Chew, E., & Zhou, J. H. (2021). Task-related brain functional network reconfigurations relate to motor recovery in chronic subcortical stroke. Scientific Reports, 11(1), Article 8442. https://doi.org/10.1038/s41598-021-87789-5
Duncan, P. W., Lai, S. M., & Keighley, J. (2000). Defining post-stroke recovery: Implications for design and interpretation of drug trials. Neuropharmacology, 39(5), 835–841. https://doi.org/10.1016/s0028-3908(00)00003-4
Evans, J. R., Dellinger, M. B., & Russell, H. L. (Eds.). (2019). Neurofeedback: The first fifty years (1st ed.). Academic Press.
Feigin, V. L., Norrving, B., George, M. G., Foltz, J. L., Roth, G. A., & Mensah, G. A. (2016). Prevention of stroke: A strategic global imperative. Nature Reviews Neurology, 12(9), 501–512. https://doi.org/10.1038/nrneurol.2016.107
Feigin, V. L., Norrving, B., & Mensah, G. A. (2017). Global burden of stroke. Circulation Research, 120(3), 439–448. https://doi.org/10.1161/CIRCRESAHA.116.308413
Fornito, A., Zalesky, A., & Breakspear, M. (2015). The connectomics of brain disorders. Nature Reviews Neuroscience, 16(3), 159–172. https://doi.org/10.1038/nrn3901
Fovet, T., Jardri, R., & Linden, D. (2015). Current issues in the use of fMRI-based neurofeedback to relieve psychiatric symptoms. Current Pharmaceutical Design, 21(23), 3384–3394. https://doi.org/10.2174/1381612821666150619092540
Friston, K. J. (2011). Functional and effective connectivity: A review. Brain Connectivity, 1(1), 13–36. https://doi.org/10.1089/brain.2011.0008
Gauthier, C. J., & Fan, A. P. (2019). BOLD signal physiology: Models and applications. NeuroImage, 187, 116–127. https://doi.org/10.1016/j.neuroimage.2018.03.018
Golanov, E. V., Yamamoto, S., & Reis, D. J. (1994). Spontaneous waves of cerebral blood flow associated with a pattern of electrocortical activity. American Journal of Physiology, 266(1 Pt. 2), R204–R214. https://doi.org/10.1152/ajpregu.1994.266.1.R204
Guggisberg, A. G., Koch, P. J., Hummel, F. C., & Buetefisch, C. M. (2019). Brain networks and their relevance for stroke rehabilitation. Clinical Neurophysiology, 130(7), 1098–1124. https://doi.org/10.1016/j.clinph.2019.04.004
Herrmann, C. S., & Debener, S. (2008). Simultaneous recording of EEG and BOLD responses: A historical perspective. International Journal of Psychophysiology, 67(3), 161–168. https://doi.org/10.1016/j.ijpsycho.2007.06.006
Huster, R. J., Debener, S., Eichele, T., & Herrmann, C. S. (2012). Methods for simultaneous EEG-fMRI: An introductory review. The Journal of Neuroscience, 32(18), 6053–6060. https://doi.org/10.1523/JNEUROSCI.0447-12.2012
Ives, J. R., Warach, S., Schmitt, F., Edelman, R. R., & Schomer, D. L. (1993). Monitoring the patient’s EEG during echo planar MRI. Electroencephalography and Clinical Neurophysiology, 87(6), 417–420. https://doi.org/10.1016/0013-4694(93)90156-p
Keynan, J. N., Cohen, A., Jackont, G., Green, N., Goldway, N., Davidov, A., Meir-Hasson, Y., Raz, G., Intrator, N., Fruchter, E., Ginat, K., Laska, E., Cavazza, M., & Hendler, T. (2019). Electrical fingerprint of the amygdala guides neurofeedback training for stress resilience. Nature Human Behaviour, 3(1), 63–73. https://doi.org/10.1038/s41562-018-0484-3
Keynan, J. N., Meir-Hasson, Y., Gilam, G., Cohen, A., Jackont, G., Kinreich, S., Ikar, L., Or-Borichev, A., Etkin, A., Gyurak, A., Klovatch, I., Intrator, N., & Hendler, T. (2016). Limbic activity modulation guided by functional magnetic resonance imaging-inspired electroencephalography improves implicit emotion regulation. Biological Psychiatry, 80(6), 490–496. https://doi.org/10.1016/j.biopsych.2015.12.024
Kim, D. H., & Kang, H. (2022). Changes in bihemispheric structural connectivity following middle cerebral artery infarction. Journal of Personalized Medicine, 12(1), 81. https://doi.org/10.3390/jpm12010081
Koush, Y., Rosa, M. J., Robineau, F., Heinen, K., Rieger, S. W., Weiskopf, N., Vuilleumier, P., Van De Ville, D., & Scharnowski, F. (2013). Connectivity-based neurofeedback: Dynamic causal modeling for real-time fMRI. NeuroImage, 81, 422–430. https://doi.org/10.1016/j.neuroimage.2013.05.010
Kwakkel, G., & Kollen, B. J. (2013). Predicting activities after stroke: What is clinically relevant? International Journal of Stroke, 8(1), 25–32. https://doi.org/10.1111/j.1747-4949.2012.00967.x
Larivière, S., Ward, N. S., & Boudrias, M.-H. (2018). Disrupted functional network integrity and flexibility after stroke: Relation to motor impairments. NeuroImage: Clinical, 19, 883–891. https://doi.org/10.1016/j.nicl.2018.06.010
Lecrux, C., & Hamel, E. (2016). Neuronal networks and mediators of cortical neurovascular coupling responses in normal and altered brain states. Philosophical Transactions of the Royal Society of B, Biological Sciences, 371(1705), Article 20150350. https://doi.org/10.1098/rstb.2015.0350
Li, W., Li, Y., Zhu, W., & Chen, X. (2014). Changes in brain functional network connectivity after stroke. Neural Regeneration Research, 9(1), 51–60. https://doi.org/10.4103/1673-5374.125330
Liew, S.-L., Rana, M., Cornelsen, S., Fortunato De Barros Filho, M., Birbaumer, N., Sitaram, R., Cohen, L. G., & Soekadar, S. R. (2016). Improving motor corticothalamic communication after stroke using real-time fMRI connectivity-based neurofeedback. Neurorehabilitation and Neural Repair, 30(7), 671–675. https://doi.org/10.1177/1545968315619699
Lioi, G., Butet, S., Fleury, M., Bannier, E., Lécuyer, A., Bonan, I., & Barillot, C. (2020). A multi-target motor imagery training using bimodal EEG-fMRI neurofeedback: A pilot study in chronic stroke patients. Frontiers in Human Neuroscience, 14, Article 37. https://doi.org/10.3389/fnhum.2020.00037
Lioi, G., Fleury, M., Butet, S., Lécuyer, A., Barillot, C., & Bonan, I. (2018). Bimodal EEG-fMRI neurofeedback for stroke rehabilitation: A case report. Annals of Physical and Rehabilitation Medicine, 61(Suppl), e482–e483. https://doi.org/10.1016/j.rehab.2018.05.1127
Lopes da Silva, F. (2013). EEG and MEG: Relevance to neuroscience. Neuron, 80(5), 1112–1128. https://doi.org/10.1016/j.neuron.2013.10.017
Luu, P., Tucker, D. M., Englander, R., Lockfeld, A., Lutsep, H., & Oken, B. (2001). Localizing acute stroke-related EEG changes: Assessing the effects of spatial undersampling. Journal of Clinical Neurophysiology, 18(4), 302–317. https://doi.org/10.1097/00004691-200107000-00002
Mano, M., Lécuyer, A., Bannier, E., Perronnet, L., Noorzadeh, S., & Barillot, C. (2017). How to build a hybrid neurofeedback platform combining EEG and fMRI. Frontiers in Neuroscience, 11, Article 140. https://doi.org/10.3389/fnins.2017.00140
Marek, S., & Dosenbach, N. U. F. (2018). The frontoparietal network: Function, electrophysiology, and importance of individual precision mapping. Dialogues in Clinical Neuroscience, 20(2), 133–140. https://doi.org/10.31887/DCNS.2018.20.2/smarek
Matthews, P. M., & Jezzard, P. (2004). Functional magnetic resonance imaging. Journal of Neurology, Neurosurgery, and Psychiatry, 75(1), 6–12.
Mehler, D. M. A., Williams, A. N., Krause, F., Lührs, M., Wise, R. G., Turner, D. L., Linden, D. E. J., & Whittaker, J. R. (2019). The BOLD response in primary motor cortex and supplementary motor area during kinesthetic motor imagery based graded fMRI neurofeedback. NeuroImage, 184, 36–44. https://doi.org/10.1016/j.neuroimage.2018.09.007
Mehler, D. M. A., Williams, A. N., Whittaker, J. R., Krause, F., Lührs, M., Kunas, S., Wise, R. G., Shetty, H. G. M., Turner, D. L., & Linden, D. E. J. (2020). Graded fMRI neurofeedback training of motor imagery in middle cerebral artery stroke patients: A preregistered proof-of-concept study. Frontiers in Human Neuroscience, 14, Article 226. https://doi.org/10.3389/fnhum.2020.00226
Meir-Hasson, Y., Keynan, J. N., Kinreich, S., Jackont, G., Cohen, A., Podlipsky-Klovatch, I., Hendler, T., & Intrator, N. (2016). One-class FMRI-inspired EEG model for self-regulation training. PLoS ONE, 11(5), Article e0154968. https://doi.org/10.1371/journal.pone.0154968
Meir-Hasson, Y., Kinreich, S., Podlipsky, I., Hendler, T., & Intrator, N. (2014). An EEG finger-print of fMRI deep regional activation. NeuroImage, 102(Pt. 1), 128–141. https://doi.org/10.1016/j.neuroimage.2013.11.004
Mel’nikov, M. Y., Shtark, M. B., Savelov, A. A., & Bruhl, A. (2017). Real time fuctional magnetic resonance imaging biofeedback: A new generation of neurotherapy. Zhurnal Vysshei Nervnoi Deiatelnosti imeni I. P. Pavlova, 67(1), 3–32.
Menon, V. (2011). Large-scale brain networks and psychopathology: A unifying triple network model. Trends in Cognitive Sciences, 15(10), 483–506. https://doi.org/10.1016/j.tics.2011.08.003
Miller, K. L., Luh, W.-M., Liu, T. T., Martinez, A., Obata, T., Wong, E. C., Frank, L. R., & Buxton, R. B. (2001). Nonlinear temporal dynamics of the cerebral blood flow response. Human Brain Mapping, 13(1), 1–12. https://doi.org/10.1002/hbm.1020
Molnar-Szakacs, I., & Uddin, L. Q. (2013). Self-processing and the default mode network: Interactions with the mirror neuron system. Frontiers in Human Neuroscience, 7, Article 571. https://doi.org/10.3389/fnhum.2013.00571
Morgenroth, E., Saviola, F., Gilleen, J., Allen, B., Lührs, M., W Eysenck, M., & Allen, P. (2020). Using connectivity-based real-time fMRI neurofeedback to modulate attentional and resting state networks in people with high trait anxiety. NeuroImage: Clinical, 25, Article 102191. https://doi.org/10.1016/j.nicl.2020.102191
Nudo R. J. (2003). Functional and structural plasticity in motor cortex: Implications for stroke recovery. Physical Medicine and Rehabilitation Clinics of North America, 14(1 Suppl.), S57–S76. https://doi.org/10.1016/s1047-9651(02)00054-2
Nudo, R. J., Wise, B. M., SiFuentes, F., & Milliken, G. W. (1996). Neural substrates for the effects of rehabilitative training on motor recovery after ischemic infarct. Science, 272(5269), 1791–1794. https://doi.org/10.1126/science.272.5269.1791
Ogawa, S., & Lee, T.-M. (1990). Magnetic resonance imaging of blood vessels at high fields: In vivo and in vitro measurements and image simulation. Magnetic Resonance in Medicine, 16(1), 9–18. https://doi.org/10.1002/mrm.1910160103
Ogawa, S., Menon, R. S., Kim, S. G., & Ugurbil, K. (1998). On the characteristics of functional magnetic resonance imaging of the brain. Annual Review of Biophysics and Biomolecular Structure, 27, 447–474. https://doi.org/10.1146/annurev.biophys.27.1.447
Paret, C., Goldway, N., Zich, C., Keynan, J. N., Hendler, T., Linden, D., & Cohen Kadosh, K. (2019). Current progress in real-time functional magnetic resonance-based neurofeedback: Methodological challenges and achievements. NeuroImage, 202, 116107. https://doi.org/10.1016/j.neuroimage.2019.116107
Petersen, S. E., & Sporns, O. (2015). Brain networks and cognitive architectures. Neuron, 88(1), 207–219. https://doi.org/10.1016/j.neuron.2015.09.027
Philiastides, M. G., Tu, T., & Sajda, P. (2021). Inferring macroscale brain dynamics via fusion of simultaneous EEG-fMRI. Annual Review of Neuroscience, 44, 315–334. https://doi.org/10.1146/annurev-neuro-100220-093239
Raichle, M. E., MacLeod, A. M., Snyder, A. Z., Powers, W. J., Gusnard, D. A., & Shulman, G. L. (2001). A default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America, 98(2), 676–682. https://doi.org/10.1073/pnas.98.2.676
Rance, M., Ruttorf, M., Nees, F., Schad, L. R., & Flor, H. (2014). Neurofeedback of the difference in activation of the anterior cingulate cortex and posterior insular cortex: Two functionally connected areas in the processing of pain. Frontiers in Behavioral Neuroscience, 8, Article 357. https://doi.org/10.3389/fnbeh.2014.00357
Ritter, P., & Villringer, A. (2006). Simultaneous EEG-fMRI. Neuroscience & Biobehavioral Reviews, 30(6), 823–838. https://doi.org/10.1016/j.neubiorev.2006.06.008
Rowe, J. B. (2010). Connectivity analysis is essential to understand neurological disorders. Frontiers in Systems Neuroscience, 4, Article 144. https://doi.org/10.3389/fnsys.2010.00144
Rudnev, V., Melnikov, M., Savelov, A., Shtark, M., & Sokhadze, E. M. (2021). fMRI-EEG fingerprint regression model for motor cortex. NeuroRegulation, 8(3), 162–172. https://doi.org/10.15540/nr.8.3.162
Savelov, A. A., Shtark, M. B., Kozlova, L. I., Verevkin, E. G., Petrovskii, E. D., Pokrovskii, M. A., Rudych, P. D., & Tsyrkin, G. M. (2019). Dynamics of interactions between cerebral networks derived from fMRI data and motor rehabilitation during stokes. Bulletin of Experimental Biology and Medicine, 166(3), 399–403. https://doi.org/10.1007/s10517-019-04359-6
Savelov, A. A., Shtark, M. B., Mel’nikov, M. E., Kozlova, L. I., Bezmaternykh, D. D., Verevkin, E. G., Petrovskii, E. D., Pokrovskii, M. A., Tsirkin, G. M., & Rudych, P. D. (2019a). Prospects of synchronous fMRI-EEG recording as the basis for neurofeedback (exemplified on patient with stroke sequelae). Bulletin of Experimental Biology and Medicine, 166(3), 390–393. https://doi.org/10.1007/s10517-019-04357-8
Savelov, A. A., Shtark, M. B., Mel’nikov, M. E., Kozlova, L. I., Bezmaternykh, D. D., Verevkin, E. G., Petrovskii, E. D., Pokrovskii, M. A., Tsirkin, G. M., & Rudych, P. D. (2019b). Dynamics of fMRI and EEG parameters in a stroke patient assessed during a neurofeedback course focused on Brodmann area 4 (M1). Bulletin of Experimental Biology and Medicine, 166(3), 394–398. https://doi.org/10.1007/s10517-019-04358-7
Schaechter, J. D., Moore, C. I., Connell, B. D., Rosen, B. R., & Dijkhuizen, R. M. (2006). Structural and functional plasticity in the somatosensory cortex of chronic stroke patients. Brain, 129(10), 2722–2733. https://doi.org/10.1093/brain/awl214
Scharnowski, F., Veit, R., Zopf, R., Studer, P., Bock, S., Diedrichsen, J., Goebel, R., Mathiak, K., Birbaumer, N., & Weiskopf, N. (2015). Manipulating motor performance and memory through real-time fMRI neurofeedback. Biological Psychology, 108, 85–97. https://doi.org/10.1016/j.biopsycho.2015.03.009
Shtark, M. B. (2019). Neurofeedback: A scarce resource at the mental market. In J. R. Evans, M. B. Dellinger, & H. L. Russell (Eds.), Neurofeedback: The first fifty years (pp. 353–358). Academic Press. https://doi.org/10.1016/B978-0-12-817659-7.00046-4
Shtark, M. B., Korostyshevskaia, A. M., Rezakova, M. V., & Savelov, A. A. (2012). Functional magnetic resonance imaging and neuroscience Uspekhi Fiziologicheskikh Nauk, 43(1), 3–29.
Shtark, M. B., Verevkin, E. G., Kozlova, L. I., Mazhirina, K. G., Pokrovskii, M. A., Petrovskii, E. D., Savelov, A. A., Starostin, A. S., & Yarosh, S. V. (2015). Synergetic fMRI-EEG brain mapping in alpha-rhythm voluntary control mode. Bulletin of Experimental Biology and Medicine, 158(5), 644–649. https://doi.org/10.1007/s10517-015-2827-7
Siegel, J. S., Ramsey, L. E., Snyder, A. Z., Metcalf, N. V., Chacko, R. V., Weinberger, K., Baldassarre, A., Hacker, C. D., Shulman, G. L., & Corbetta, M. (2016). Disruptions of network connectivity predict impairment in multiple behavioral domains after stroke. Proceedings of the National Academy of Sciences of the United States of America, 113(30), E4367–E4376. https://doi.org/10.1073/pnas.1521083113
Sitaram, R., Veit, R., Stevens, B., Caria, A., Gerloff, C., Birbaumer, N., & Hummel, F. (2012). Acquired control of ventral premotor cortex activity by feedback training: An exploratory real-time FMRI and TMS study. Neurorehabilitation and Neural Repair, 26(3), 256–265. https://doi.org/10.1177/1545968311418345
Sokolova, O. O., Shtark, M. B., & Lisachev, P. D. (2010). Neuronal plasticity and gene expression. Uspekhi Fiziologicheskikh Nauk, 41(1), 26–44.
Sorger, B., Kamp, T., Weiskopf, N., Peters, J. C., & Goebel, R. (2018). When the brain takes ‘BOLD’ steps: Real-time fMRI neurofeedback can further enhance the ability to gradually self-regulate regional brain activation. Neuroscience, 378, 71–88. https://doi.org/10.1016/j.neuroscience.2016.09.026
Spampinato, D. A., Block, H. J., & Celnik, P. A. (2017). Cerebellar–M1 connectivity changes associated with motor learning are somatotopic specific. Journal of Neuroscience, 37(9), 2377–2386. https://doi.org/10.1523/JNEUROSCI.2511-16.2017
Sporns, O., & Honey, C. J. (2006). Small worlds inside big brains. Proceedings of the National Academy of Sciences of the United States of America, 103(51), 19219–19220. https://doi.org/10.1073/pnas.0609523103
Stoeckel, L. E., Garrison, K. A., Ghosh, S., Wighton, P., Hanlon, C. A., Gilman, J. M., Greer, S., Turk-Browne, N. B., deBettencourt, M. T., Scheinost, D., Craddock, C., Thompson, T., Calderon, V., Bauer, C. C., George, M., Breiter, H. C., Whitfield-Gabrieli, S., Gabrieli, J. D., LaConte, S. M., Hirshberg, L., … Evins, A. E. (2014). Optimizing real time fMRI neurofeedback for therapeutic discovery and development. NeuroImage: Clinical, 5, 245–255. https://doi.org/10.1016/j.nicl.2014.07.002
Sulzer, J., Haller, S., Scharnowski, F., Weiskopf, N., Birbaumer, N., Blefari, M. L., Bruehl, A. B., Cohen, L. G., deCharms, R. C., Gassert, R., Goebel, R., Herwig, U., LaConte, S., Linden, D., Luft, A., Seifritz, E., & Sitaram, R. (2013). Real-time fMRI neurofeedback: Progress and challenges. NeuroImage, 76, 386–399. https://doi.org/10.1016/j.neuroimage.2013.03.033
Ullsperger, M., & Debener, S. (Eds.). (2010). Simultaneous EEG and fMRI: Recording, analysis, and application. Oxford University Press. https://doi.org/10.1093/acprof:oso/9780195372731.001.0001
Tang, C., Zhao, Z., Chen, C., Zheng, X., Sun, F., Zhang, X., Tian, J., Fan, M., Wu, Y., & Jia, J. (2016). Decreased functional connectivity of homotopic brain regions in chronic stroke patients: A resting state fMRI study. PLoS ONE, 11(4), Article e0152875. https://doi.org/10.1371/journal.pone.0152875
van Meer, M. P. A., van der Marel, K., Wang, K., Otte, W. M., el Bouazati, S., Roeling, T. A. P., Viergever, M. A., Berkelbach van der Sprenkel, J. W., & Dijkhuizen, R. M. (2010). Recovery of sensorimotor function after experimental stroke correlates with restoration of resting-state interhemispheric functional connectivity. The Journal of Neuroscience, 30(11), 3964–3972. https://doi.org/10.1523/JNEUROSCI.5709-09.2010
Wang, C., Stebbins, G. T., Nyenhuis, D. L., deToledo-Morrell, L., Freels, S., Gencheva, E., Pedelty, L., Sripathirathan, K., Moseley, M. E., Turner, D. A., Gabrieli, J. D. E., & Gorelick, P. B. (2006). Longitudinal changes in white matter following ischemic stroke: A three-year follow-up study. Neurobiology of Aging, 27(12), 1827–1833. https://doi.org/10.1016/j.neurobiolaging.2005.10.008
Wang, L., Yu, C., Chen, H., Qin, W., He, Y., Fan, F., Zhang, Y., Wang, M., Li, K., Zang, Y., Woodward, T. S., & Zhu, C. (2010). Dynamic functional reorganization of the motor execution network after stroke. Brain, 133(4), 1224–1238. https://doi.org/10.1093/brain/awq043
Wang, W., Collinger, J. L., Perez, M. A., Tyler-Kabara, E. C., Cohen, L. G., Birbaumer, N., Brose, S. W., Schwartz, A. B., Boninger, M. L., & Weber, D. J. (2010). Neural interface technology for rehabilitation: Exploiting and promoting neuroplasticity. Physical Medicine and Rehabilitation Clinics of North America, 21(1), 157–178. https://doi.org/10.1016/j.pmr.2009.07.003
Yoo, S.-S., & Jolesz, F. A. (2002). Functional MRI for neurofeedback: Feasibility study on a hand motor task. NeuroReport, 13(11), 1377–1381. https://doi.org/10.1097/00001756-200208070-00005
Yu, X., Jiaerken, Y., Wang, S., Hong, H., Jackson, A., Yuan, L., Lou, M., Jiang, Q., Zhang, M., & Huang, P. (2020). Changes in the corticospinal tract beyond the ischemic lesion following acute hemispheric stroke: A diffusion kurtosis imaging study. Journal of Magnetic Resonance Imaging, 52(2), 512–519. https://doi.org/10.1002/jmri.27066
Yuan, K., Chen, C., Wang, X., Chu, W. C.-W., & Tong, R. K.-Y. (2021). BCI training effects on chronic stroke correlate with functional reorganization in motor-related regions: A concurrent EEG and fMRI study. Brain Sciences, 11(1), 56. https://doi.org/10.3390/brainsci11010056
Zeiler, S. R., Gibson, E. M., Hoesch, R. E., Li, M. Y., Worley, P. F., O’Brien, R. J., & Krakauer, J. W. (2013). Medial premotor cortex shows a reduction in inhibitory markers and mediates recovery in a mouse model of focal stroke. Stroke, 44(2), 483–489. https://doi.org/10.1161/STROKEAHA.112.676940
Zhang, Y., Liu, H., Wang, L., Yang, J., Yan, R., Zhang, J., Sang, L., Li, P., Wang, J., & Qiu, M. (2016). Relationship between functional connectivity and motor function assessment in stroke patients with hemiplegia: A resting-state functional MRI study. Neuroradiology, 58(5), 503–511. https://doi.org/10.1007/s00234-016-1646-5
Zhuravleva, K. V., Savelov, A. A., Korostyshevskaya, A. M., & Shtark, M. B. (2022). Diffusional characteristics of brain matter after stroke. Bulletin of Experimental Biology and Medicine, 172(4), 402–406. https://doi.org/10.1007/s10517-022-05402-9
Zotev, V., Phillips, R., Yuan, H., Misaki, M., & Bodurka, J. (2014). Self-regulation of human brain activity using simultaneous real-time fMRI and EEG neurofeedback. NeuroImage, 85(Pt. 3), 985–995. https://doi.org/10.1016/j.neuroimage.2013.04.126
Source