Posts Tagged BCI
[Abstract] Hybrid Brain-Computer Interface Controlled Soft Robotic Glove for Stroke Rehabilitation
Posted by Kostas Pantremenos in Paretic Hand, Rehabilitation robotics on April 26, 2024
Abstract:
Soft robotic glove controlled by a brain-computer interface (BCI) have demonstrated effectiveness in hand rehabilitation for stroke patients. Current systems mostly rely on static visual representations for patients to perform motor imagination (MI) tasks, resulting in lower BCI performance. Therefore, this study innovatively used MI and high-frequency steady-state visual evoked potential (SSVEP) to construct a friendly and natural hybrid BCI paradigm. Specifically, the stimulation interface sequentially presented decomposed action pictures of the left and right hands gripping a ball, with the pictures flashing at specific stimulation frequencies (left: 34 Hz, right: 35 Hz). Integrating soft robotic glove as feedback, we established a comprehensive “peripheral – central – peripheral” hand rehabilitation system to facilitate the hand rehabilitation of patients. Filter bank common spatial pattern (FBCSP) and filter bank canonical correlation analysis (FBCCA) algorithms were used to identify MI and SSVEP signals, respectively. Additionally, to fuse the features of these two signals, we proposed a novel fusion algorithm for improving the recognition accuracy of the system. The feasibility of the proposed system was validated through online experiments involving 12 healthy subjects and 9 stroke patients, achieving accuracy rates of 95.83 ± 6.83% and 63.33 ± 10.38%, respectively. The accuracy of MI and SSVEP in 12 healthy subjects reached 81.67 ± 15.63% and 95.14 ± 7.47%, both lower than the accuracy after fusion, these results confirmed the effectiveness of the proposed algorithm. The accuracy rate was more than 50% in both healthy subjects and patients, confirming the effectiveness of the proposed system.
[Review] The evolution of neuromodulation for chronic stroke: From neuroplasticity mechanisms to brain-computer interfaces – Full Text
Posted by Kostas Pantremenos in Neuroplasticity, REHABILITATION on March 1, 2024
Abstract
Stroke is one of the most common and debilitating neurological conditions worldwide. Those who survive experience motor, sensory, speech, vision, and/or cognitive deficits that severely limit remaining quality of life. While rehabilitation programs can help improve patients’ symptoms, recovery is often limited, and patients frequently continue to experience impairments in functional status. In this review, invasive neuromodulation techniques to augment the effects of conventional rehabilitation methods are described, including vagus nerve stimulation (VNS), deep brain stimulation (DBS) and brain-computer interfaces (BCIs). In addition, the evidence base for each of these techniques, pivotal trials, and future directions are explored. Finally, emerging technologies such as functional near-infrared spectroscopy (fNIRS) and the shift to artificial intelligence-enabled implants and wearables are examined. While the field of implantable devices for chronic stroke recovery is still in a nascent stage, the data reviewed are suggestive of immense potential for reducing the impact and impairment from this globally prevalent disorder.
Introduction
Due to improving techniques for treating acute stroke, more patients than ever are entering the chronic stroke phase during which motor recovery becomes significantly more challenging [1]. Approximately 34% of the global total healthcare expenditure is spent on stroke, and in the US, the economic burden of chronic stroke increases by approximately $140,000 for treatment, rehabilitation and supportive care over the course of a typical patient’s lifetime [[2], [3], [4]]. Furthermore, incidence rates of chronic stroke are projected to grow due to a global increase in population age [2]. These worrisome trends underline the crucial need for effective rehabilitation to improve quality-of-life and enable patients to recover functional ability post-stroke.
The current standard-of-care for post-stroke recovery is physical rehabilitation, which exploits the innate neuroplasticity of the brain to restore function [1,5]. Physical rehabilitation programs, especially when delivered as soon as possible after the onset of stroke, can be highly efficacious [5]. Notwithstanding, the rate of improvement in functional ability regained through physical rehabilitation tends to peak after a few months post-stroke and eventually tapers; minimal improvement is seen after 12 months and many patients remain considerably disabled. Therefore, a critical need exists for interventions that can either increase the rate of functional recovery during the early post-stroke period or that can produce meaningful functional improvement after 12 months. Given that the nature of post-stroke functional recovery is mediated by neuroplastic changes, interventions that increase or prolong neuroplasticity have been the target of recent investigations.
One such intervention is the application of electromagnetic energy to the brain in the form of neuromodulation, which has been shown to be an effective trigger for neuroplastic processes such as synaptogenesis and functional reorganization [6]. Both invasive and non-invasive modalities exist. Non-invasive modalities such as transcranial direct current stimulation (tDCS) and transcranial magnetic stimulation (TMS), have demonstrated improvement of motor function in post-stroke patients [6]. Similarly, invasive modalities such as vagus nerve stimulation (VNS) and deep brain stimulation (DBS) show great promise in improving rehabilitation in stroke patients suffering from disabling symptoms. Moreover, there has been rapid development of therapeutic neurostimulation in the form of brain-computer interfaces (BCIs), which utilizes real-time analysis of brain states to enable automatic adjustment of stimulation parameters [7]. In this paper, we will discuss the landmark trials, current applications, and future directions of the various modalities of invasive neuromodulation for stroke rehabilitation with an emphasis on VNS, DBS and BCI. […]
[WEB] The power of brain-computer interface assistive devices for motor impairments
Posted by Kostas Pantremenos in Assistive Technology on January 29, 2024

Naeem Komeilipoor, CTO of AAVAA, discusses how brain-computer interface assistive devices are working to overcome the limitations of current assistive products, and how these devices enable users with motor impairments to operate devices without difficult physical or spoken commands.
In a world that celebrates technological innovation and connectivity, it is vital to remember that not everyone enjoys the same level of access to these benefits. Globally, more than 2.5 billion people require assistive products to overcome mobility impairments and speech communication disorders, according to data from the World Health Organization (WHO). These individuals often face significant challenges in their daily lives, struggling to communicate effectively and engage with the modern, technologically advanced world.
Brain-computer interface technology has undergone a remarkable transformation over the years and is, among other things, providing a long-awaited solution to those with motor impairments. These devices are used to provide functionality to paralysed people, control devices without difficult physical or spoken demands, create the controller of the future for AR/VR environments, and even change how astronauts function in space.
In the ever-evolving landscape of accessibility technology, brain-controlled assistive devices are poised to make a significant impact, offering newfound independence and capabilities to individuals facing motor and speech-related challenges in areas such as enabling paralysed individuals to steer their wheelchairs, operate household appliances, and much more. They are ushering in an era where interaction transcends traditional physical and verbal constraints and radically transforms the daily life of these individuals.
Understanding the types of impairments
To comprehend the significance of brain-controlled assistive devices, it is essential to first define the two primary categories of motor impairments they address:
- Complete mobility impairments and paralysis. This category encompasses individuals with a diverse range of conditions, including cerebral palsy, spinal cord injuries, quadriplegia, amyotrophic lateral sclerosis (ALS), and even those who maintain control over their eye and/or facial movements, allowing for subtle gestures or slight head movements. While some may retain limited motor skills, their movement is significantly restricted, often requiring assistance with daily activities.
- Speech communication disorders with motor impairments. People in this category not only struggle with mobility but also face challenges in speech communication, including conditions such as the locked-in syndrome, neurodegenerative disorders such as cerebral palsy, and those with spinal cords and traumatic brain injuries. Existing assistive technologies fall short in providing comprehensive solutions for them.
Both categories share common problems, including their reliance on assistance for tasks most take for granted and limitations in current assistive technologies.
Existing challenges in assistive technologies
Current assistive technologies, although invaluable, have limitations that impact the quality of life for individuals with motor impairments and speech communication disorders. These challenges include:
- Limited interaction – Many assistive technologies are unable to seamlessly interact with other devices, leading to dependence on caregivers, nurses, or personal assistants for even basic tasks.
- Single device interaction – Users often struggle with the inability to interact with multiple devices simultaneously, limiting their independence and productivity.
- Eye-tracking inefficiencies – Eye-tracking technology, a common solution, suffers from inefficiencies. It requires periodic calibration and often relies on infrared light, making it sensitive to various lighting conditions, including bright sunlight. Additionally, it may not work effectively for individuals with smaller or less distinct eye features, further limiting its reliability as an assistive solution.
- Gesture recognition – Distinguishing between intentional gestures, like blinks or slight, involuntary head movements, and unintentional ones remains a challenge, often resulting in misunderstandings and causing frustration among users.
A new, innovative solution for motor impairments: Brain-computer interface assistive devices
Brain-computer interface (BCI) assistive devices are working to overcome the limitations of current assistive products. BCI assistive devices translate brain and bio-signals directly into meaningful commands for devices.
For the first time, users with motor impairments can operate devices without difficult physical or spoken commands. The technology decodes a user’s eye movements, as well as their intentions and subtle commands, including blinks, winks, tongue movements, and more.
A real-world application showcasing the power of this technology is the AAVAA Headband. This groundbreaking wearable device uniquely operates as a ‘head mouse’ capable of deciphering a user’s head and eye movements, as well as their intentions and subtle commands. This capability empowers users to manage various devices, like phones, tablets, or assistive tools, with straightforward actions like a blink, thereby reshaping accessibility for individuals with limited motor function.

BCI assistive devices empower users to interact with multiple devices simultaneously, fostering independence and efficiency. These devices remain unaffected by varying lighting conditions and exhibit the capability to accurately distinguish between intentional and unintentional gestures, thanks to their reliance on physiological cues and advanced algorithms.
They have the potential to transform the lives of individuals with motor impairments and speech communication disorders in numerous ways:
- Environmental control – Brain-controlled devices enable users to effortlessly manage their home environment through intuitive eye, blink, and head commands, enhancing daily living by promoting independence and comfort. Tasks include muting the TV, adjusting smart home devices, like speakers, or dimming the lights.
- Communication enhancement – These innovative technologies enable individuals with speech disorders to communicate fluidly by typing and speaking through simple head and eye movements, which not only strengthens their social connections but also results in significant enhancements in healthcare communication with providers. This breakthrough diminishes the necessity for intermediaries, ultimately promoting greater independence and improving the overall quality of care for these individuals.
- Mobility enhancement – BCI devices further expand their functionalities to include wheelchair control, granting individuals with physical disabilities greater mobility and independence in their everyday activities, whether it’s running errands or engaging in social interactions.
BCI sensors have the remarkable ability to blend information from the environment, brain, and body using only a hearable device. This comprehensive data collection goes beyond improving health and well-being; it offers a unique insight into one’s physiological state, potentially revolutionising healthcare practices.
Additionally, we can consider as applications of BCI technology:
- Health monitoring: BCI devices can monitor trauma and sudden movements and even fall detection, helping caregivers and medical professionals provide timely assistance. These devices can also track stress, fatigue, and heart rate, enabling more effective health management and enhancing overall well-being.
- Supporting health and social care professionals: Through the use of BCI devices, social workers can continuously monitor patients without hindering their movements or causing distractions, improving support and care.
These devices are available to users in a variety of form factors, such as hardware and earpieces, including earbuds, headphones, glasses, and headbands. The emphasis on ergonomic and user-friendly design ensures that these devices are seamlessly integrated into daily life, offering not only advanced functionality but also comfort and accessibility.
The advent of brain-computer interface assistive devices represents a beacon of hope for individuals with motor impairments and speech communication disorders. These technologies can significantly enhance their quality of life by addressing the limitations of existing solutions.
As the field of assistive technology continues to advance, we can look forward to even more transformative innovations like BCI technology that empower those in need while also making it easier for health and social care professionals to do their jobs more efficiently.
[Review] Brain–Computer Interfaces for Upper Limb Motor Recovery after Stroke: Current Status and Development Prospects – Full Text
Posted by Kostas Pantremenos in Paretic Hand on January 17, 2024
Brain–computer interfaces (BCIs) are a group of technologies that allow mental training with feedback for post-stroke motor recovery. Varieties of these technologies have been studied in numerous clinical trials for more than 10 years, and their construct and software are constantly being improved. Despite the positive treatment results and the availability of registered medical devices, there are currently a number of problems for the wide clinical application of BCI technologies. This review provides information on the most studied types of BCIs and its training protocols and describes the evidence base for the effectiveness of BCIs for upper limb motor recovery after stroke. The main problems of scaling this technology and ways to solve them are also described.
Introduction
Brain–computer interface (BCI) is a technology that allows to convert data on the electrical or metabolic activity of the brain into control signals for an external technical device. In post-stroke rehabilitation, BCI is used to provide feedback to a patient during motor imagery training [1–3]. The scientific justification for this method has been the data on the positive effect of the motor imagery process on neuroplasticity due to activation of motor structures of the central nervous system (CNS) [4–8]. By providing feedback during motor imagery, the BCI systems enhance the effectiveness of such training sessions [9]. In general, training with the use of the BCI technology in patients after stroke includes the following processes: a patient is asked to mentally perform a movement of the paralyzed limb; the BCI technology using non-invasive sensors records brain signals accompanying the mental performance of the task; in real time, these signals are recognized and converted into a control command for an external device; the patient is provided with feedback on the quality of the mental task performance using the external device [10].
To date, at least 20 randomized controlled trials (RCTs) on the use of BCI for upper limb motor recovery after stroke are known worldwide, and 11 systematic reviews, 8 of which are accompanied by a meta-analysis, have been published on this topic between 2019 and 2023 [11–21]. Foreign and domestic manufacturers have developed several medical devices for use in clinical practice of post-stroke rehabilitation [22–25].
In Russia, clinical trials of BCI after stroke first began in 2011 at Research Center of Neurology (Moscow, Russia) [26, 27]. In a subsequent multicentre RCT, it was shown that a course of training with the BCI–exoskeleton complex improved the rehabilitation results of patients with focal brain damage in terms of hand motor recovery [28]. The proven technology was subsequently registered as a medical device and is currently used in a number of clinical centres [24, 29].
Despite the extensive evidence base and the availability of ready-made BCI technologies, there are currently some limitations to their widespread use in post-stroke rehabilitation, and further research and development is underway [30–37].
The aim of this review is to analyse scientific articles devoted to the study of the use of BCI technologies in post-stroke upper limb paresis, to outline the main problems and prospects for further development in this field.
Literature search methodology
Articles from peer-reviewed, full-text, open access scientific journals on the use of non-invasive BCIs for upper limb motor recovery after stroke were selected for analysis. The search query was formulated according to the rules of the MEDLINE bibliographic database: ((brain–computer[tiab] OR brain–machine[tiab] OR neural interfac*[tiab]) OR “Brain–Computer interfaces”[Mesh]) AND stroke[mh] AND (upper extremity[tiab] OR hand[tiab] OR arm[tiab]). Additionally, a literature search was conducted in the eLIBRARY.RU system using the key words “brain–computer interface”, “neurocomputer interface”, “neurointerface”. The date of the search was July 3, 2023.
Varieties of brain–computer interface systems and their application after stroke
All BCIs used in research or in the practice of post-stroke rehabilitation have distinctive features (see the Figure). The training protocols and BCI models studied in RCTs differ in the control paradigm of the interface, the type of signal recorded, the online signal processing algorithm, and the type of external technical device for providing feedback.

[Abstract] Transcranial Direct Current Stimulation and Brain–Computer Interfaces for Improving Post-Stroke Recovery: A Systematic Review and Meta-Analysis
Posted by Kostas Pantremenos in REHABILITATION, tDCS/rTMS on November 8, 2023
Abstract
Objective
This study aimed to evaluate the effectiveness of transcranial direct current stimulation associated with brain–computer interface in stroke patients.
Data sources
The PubMed, Central, PEDro, Web of Science, SCOPUS, PsycINFO Ovid, CINAHL EBSCO, EMBASE, and ScienceDirect databases were searched from inception to April 2023 for randomized controlled studies reporting the effects of active transcranial direct current stimulation associated with brain–computer interface to a transcranial direct current stimulation sham associated with brain–computer interface condition on the outcome measure (motor performance and functional independence).
Review methods
We searched for full-text articles which had investigated the effect of transcranial direct current stimulation associated with brain–computer interface on motor performance in the upper extremities in stroke patients. The standardized mean differences derived from the change in scores between pretreatment and post-treatment were adopted as the effect size measure, with a 95% confidence interval. Possible sources of heterogeneity were analyzed by performing subgroup analyses in order to examine the moderating effects for one variable: the level of injury severity.
Results
Nine studies were included in the qualitative synthesis and the meta-analysis. The findings of the conducted analyses indicated there is not enough evidence to suggest that active transcranial direct current stimulation associated with brain–computer interface is more efficient in motor performance and functional independence when compared to sham transcranial direct current stimulation associated with brain–computer interface or brain–computer interface alone. In addition, the quality of evidence was rated very low. A subgroup analysis was performed for the motor performance outcome considering the injury severity level.
Conclusion
We found evidence that transcranial direct current stimulation associated with brain–computer interface was not more beneficial than sham transcranial direct current stimulation associated with brain–computer interface or brain–computer interface alone.
Get full access to this article
View all access and purchase options for this article.
References
1. Boden-Albala B, Appleton N, Schram B. Stroke epidemiology and prevention. In: Wilson R, Raghavan P (eds) Stroke rehabilitation. St. Louis, MO: Elsevier, 2019, pp.1–21.
2. Lee JJ. Lower limb impairments after stroke. In: Wilson R, Raghavan P (eds) Stroke rehabilitation. St. Louis, MO: Elsevier, 2019, pp.123–132.
3. Feng W, et al. Trans cranial direct current stimulation for poststroke motor recovery: challenges and opportunities. PM&R 2018; 10: S157–S164.
4. Elsner B, Kugler J, Pohl M, et al. Transcranial direct current stimulation (tDCS) for improving activities of daily living, and physical and cognitive functioning, in people after stroke. Cochrane Database Syst Rev 2016; 3: 1–168.
5. Fregni F, et al. Evidence-based guidelines and secondary meta-analysis for the use of transcranial direct current stimulation in neurological and psychiatric disorders. Int J Neuropsychopharmacol 2021; 24: 256–313.
6. Shih JJ, Krusienski DJ, Wolpaw JR. Brain-computer interfaces in medicine. Mayo Clin Proc 2012; 87: 268–279.
7. van Dokkum LEH, Ward T, Laffont I. Brain computer interfaces for neurorehabilitation—its current status as a rehabilitation strategy post-stroke. Ann Phys Rehabil Med 2015; 58: 3–8.
8. Danzl M, Chelette K, Lee K, et al. Brain stimulation paired with novel locomotor training with robotic gait orthosis in chronic stroke: a feasibility study. NeuroRehabilitation 2013; 33: 67–76.
9. Hesse S, et al. Combined transcranial direct current stimulation and robot-assisted arm training in subacute stroke patients: an exploratory, randomized multicenter trial. Neurorehabil Neural Repair 2011; 25: 838–846.
10. Cheng HJ, et al. Task-related brain functional network reconfigurations relate to motor recovery in chronic subcortical stroke. Sci Rep 2021; 11: 1–12.
11. Ang KK, et al. Facilitating effects of transcranial direct current stimulation on motor imagery brain-computer interface with robotic feedback for stroke rehabilitation. Arch Phys Med Rehabil 2015; 96: S79–S87.
12. Chew E, et al. Using transcranial direct current stimulation to augment the effect of motor imagery-assisted brain-computer interface training in chronic stroke patients—cortical reorganization considerations. Front Neurol 2020; 11: 948.
13. Hong X, et al. Brain plasticity following MI-BCI training combined with tDCS in a randomized trial in chronic subcortical stroke subjects: a preliminary study. Sci Rep 2017; 7: 9222.
14. Hu M, et al. Brain functional changes in stroke following rehabilitation using brain-computer interface-assisted motor imagery with and without tDCS: a pilot study. Front Hum Neurosci 2021; 15: 692304.
15. Mane R, et al. Prognostic and monitory EEG-biomarkers for BCI upper-limb stroke rehabilitation. IEEE Trans Neural Syst Rehabil Eng 2019; 27: 1654–1664.
16. Kasashima-Shindo Y, et al. Brain-computer interface training combined with transcranial direct current stimulation in patients with chronic severe hemiparesis: proof of concept study. J Rehabil Med 2015; 47: 318–324.
17. Straudi S, et al. tDCS and robotics on upper limb stroke rehabilitation: effect modification by stroke duration and type of stroke. Biomed Res Int 2016; 2016: 5068127.
18. Comino-Suárez N, et al. Transcranial direct current stimulation combined with robotic therapy for upper and lower limb function after stroke: a systematic review and meta-analysis of randomized control trials. J Neuroeng Rehabil 2021; 18: 148.
19. Guerra A, López-Alonso V, Cheeran B, et al. Solutions for managing variability in non-invasive brain stimulation studies. Neurosci Lett 2017; 719: 133332.
20. Guerra A, López-Alonso V, Cheeran B,. et al. Variability in non-invasive brain stimulation studies: reasons and results. Neurosci Lett 2020; 719: 133330.
21. Ang KK, Guan C. Brain-computer interface in stroke rehabilitation. J Comput Sci Eng 2013; 7: 139–146.
22. Bai Z, Fong KNK, Zhang JJ, et al. Immediate and long-term effects of BCI-based rehabilitation of the upper extremity after stroke: a systematic review and meta-analysis. J Neuroeng Rehabil 2020; 17: 1.
[ARTICLE ] Brain–computer interface treatment for gait rehabilitation in stroke patients – Full Text
Posted by Kostas Pantremenos in Gait Rehabilitation - Foot Drop on October 29, 2023
The use of Brain–Computer Interfaces (BCI) as rehabilitation tools for chronically ill neurological patients has become more widespread. BCIs combined with other techniques allow the user to restore neurological function by inducing neuroplasticity through real-time detection of motor-imagery (MI) as patients perform therapy tasks. Twenty-five stroke patients with gait disability were recruited for this study. Participants performed 25 sessions with the MI-BCI and assessment visits to track functional changes during the therapy. The results of this study demonstrated a clinically significant increase in walking speed of 0.19 m/s, 95%CI [0.13–0.25], p < 0.001. Patients also reduced spasticity and improved their range of motion and muscle contraction. The BCI treatment was effective in promoting long-lasting functional improvements in the gait speed of chronic stroke survivors. Patients have more movements in the lower limb; therefore, they can walk better and safer. This functional improvement can be explained by improved neuroplasticity in the central nervous system.
1. Introduction
Stroke is one of the main causes of mortality and long-term disability worldwide. Functional deficit of the lower limb is the most common paresis after a stroke. Stroke patients rarely fully recover after months or even years of therapy and other treatment, leaving them with permanent impairment. Many of these patients never regain the ability to walk well enough to perform all their daily activities (Hesse et al., 2008; Mehrholz et al., 2017). Gait recovery is one of the major therapy goals in rehabilitation programs for stroke patients and many methods for gait analysis and rehabilitation have been developed (Mehrholz et al., 2017). Weakened muscle tone is another common challenge in motor rehabilitation. Therapies such as active foot drop exercises, electromechanically assisted therapy and treadmill therapy are usually limited to patients with mild or moderate impairment (Mills et al., 2011; Mehrholz et al., 2017).
A 2018 study (Mehrholz et al., 2018) conducted a network meta-analysis based on 95 publications out of 44.567 that were considered. In this study, 4.458 patients were included, and the effectiveness of the most common interventions for gait rehabilitation after stroke was analyzed. The interventions where classified in five groups: (1) No walking training, (2) Conventional walking training (walking on the floor, preparatory exercises in a sitting position, balance training etc. without technical aids and without treadmill training or electromechanical-assisted training), (3) Treadmill training without or with body-weight support, (4) Treadmill training with or without a walking speed paradigm, (5) Electromechanical-assisted training with end-effector devices or exoskeletons. For the primary endpoint of walking speed, end-effector-assisted training (EGAIT_EE) achieved significantly greater improvements than conventional walking rehabilitation (mean difference [MD] = 0.16 m/s, 95% CI = [0.04, 0.28]). None of the other interventions improved walking speed significantly.
Functional electrical stimulation (FES) has also been used in motor rehabilitation therapy over the last few decades. Passive FES therapy can reduce muscle spasms and shorten the term of motor recovery (Hong et al., 2018). Passive therapies such as continuous passive motion or cycling therapy have been employed for patients and showed functional improvements in previous studies (Janssen et al., 2008; Yeh et al., 2010; Ambrosini et al., 2011). However, they do not include devices or systems to monitor the patient’s active engagement in the therapy.
Today, Brain-Computer Interfaces (BCIs) can provide an objective tool for measuring Motor Imagery (MI), creating new possibilities for “closed-loop” feedback (Wolpaw and Wolpaw, 2012). Closed-loop feedback depends on sensing the desired mental activity and is possible with MI-based BCIs, which could significantly improve rehabilitation therapy outcomes (Ortner et al., 2012; Cho et al., 2016; Cantillo-Negrete et al., 2018; Irimia et al., 2018).
MI-based BCIs have been employed in rehabilitation training for stroke patients to fill the gap between patients’ expectations and therapy outcomes. In conventional rehabilitation therapies, patients are often asked to try to move the paretic limb, or to imagine moving it, while a FES, physiotherapist and/or robotic device helps them to perform the desired movement. Their feedback is often provided when the users are not performing the required mental activity. There is no objective way to determine whether patients who cannot move are actively performing the desired motor imagery (MI) task and thus producing concordant neural activation. Its efficacy has been shown in multiple studies implementing exoskeleton, orthosis or robots which induce passive movement of their affected limbs (Ramos-Murguialday et al., 2013; Ono et al., 2014; Ang et al., 2015). During repetitive neurofeedback training sessions, even patients with severe impairment could complete the sensorimotor loop in their brains linking coherent sensory feedback with motor intention (Cho et al., 2016; Pichiorri et al., 2017; Irimia et al., 2018).
This concurrent sensory feedback with motor intention is an important factor for motor recovery (Ortner et al., 2012; Bolognini et al., 2016; Pichiorri et al., 2017; Cantillo-Negrete et al., 2018; Irimia et al., 2018). Concurrent feedback based on users’ intention may help them learn mental strategies associated with movement and BCI use, which can affect results (Neuper et al., 2005; Neuper and Allison, 2014). Neural networks are strengthened when the presynaptic and postsynaptic neurons are both active. In conventional therapies, when patients receive feedback while they are not performing MI, these two neuronal networks are not simultaneously firing. This dissociation between motor commands and sensory feedback may explain why the therapy does not significantly induce the reorganization of the patients’ brains around their lesioned area. Non-simultaneous, dissociated feedback cannot underlie the Hebbian learning between two neuronal populations that underlies the desired improvements from rehabilitation (Mayford et al., 2012; Wolpaw and Wolpaw, 2012). Thus, conventional therapies may sometimes fail because they rely on open-loop feedback.
This clinical trial investigated the impact of combining BCI technology with MI and FES feedback for motor recovery of the lower limbs. The patients’ real-time sensory feedback depended on their movement intention. We explore the relationship between the proposed rehabilitation method and rehabilitation results, including changes in walking speed. Patients who use the training mode may have better motor outcomes, and these outcomes will be compared with those from patients who had EGAIT_EE therapy. […]

[NEWS] University of Houston Expert Creates Portable EEG Headset for Stroke Rehab
Posted by Kostas Pantremenos in REHABILITATION on September 15, 2023

A new low-cost, portable EEG brain-computer interface that connects the brain of stroke patients to powered exoskeletons for rehabilitation purposes has been validated and tested at the University of Houston.
“We designed and validated a wireless, easy-to-use, mobile, dry-electrode headset for scalp electroencephalography (EEG) recordings for closed-loop brain–computer (BCI) interface and internet-of-things (IoT) applications,” report professor Jose Luis Contreras-Vidal, Hugh Roy, and Lillie Cranz Cullen Distinguished Professor of electrical and computer engineering, in the journal Sensors. Contreras-Vidal is an international pioneer in noninvasive brain-machine interfaces and robotic device inventions.
An EEG-based brain-computer interface (BCI) is a system that provides a pathway between the brain and external devices by interpreting EEG. In other words, the device reads your mind, interpreting the brain’s activity to initiate robotic movement. Brain-machine interfaces based on scalp EEG also have the potential to promote cortical plasticity following stroke, which has been shown to improve motor recovery outcomes.
The adjustable headset, designed from commercial off-the-shelf components, can accommodate 90% of the population. There is a patent pending on both the BCI algorithm and the self-positioning dry electrode bracket allowed for vertical self-positioning while parting the user’s hair to ensure contact of the electrode with the scalp.
“We used a multi-pronged approach that balanced interoperability, cost, portability, usability, form factor, reliability, and closed-loop operation,” says Contreras-Vidal.
In the current prototype, five EEG electrodes were incorporated in the electrode bracket spanning the sensorimotor cortices and three skin sensors were included to measure eye movement and blinks. An inertial movement unit, measuring head motion, allows for a portable brain-body imaging system for BCI applications.
“Most commercial EEG-based BCI systems are tethered to immobile processing hardware or require complex programming or set-up, making them difficult to deploy outside of the clinic or laboratory without technical assistance or extensive training. A portable and wireless BCI system is highly preferred so it can be used outside lab in clinical and non-clinical mobile applications at home, work, or play,” says Contreras-Vidal.
The invention was designed to solve an array of needs.
“Current commercial EEG amplifiers and BCI headsets are prohibitively expensive, lack interoperability, or fail to provide a high signal quality or closed-loop operation, which are vital for BCI applications,” Contreras-Vidal says.
About the University of Houston
The University of Houston is a Carnegie-designated Tier One public research university recognized with a Phi Beta Kappa chapter for excellence in undergraduate education. UH serves the globally competitive Houston and Gulf Coast Region by providing world-class faculty, experiential learning, and strategic industry partnerships. Located in the nation’s fourth-largest city and one of the most ethnically and culturally diverse regions in the country, UH is a federally designated Hispanic- and Asian-American-Serving institution with enrollment of more than 47,000 students.
[Abstract + References] Transcranial Direct Current Stimulation and Brain–Computer Interfaces for Improving Post-Stroke Recovery: A Systematic Review and Meta-Analysis
Posted by Kostas Pantremenos in REHABILITATION, tDCS/rTMS on September 14, 2023
Abstract
Objective
This study aimed to evaluate the effectiveness of transcranial direct current stimulation associated with brain–computer interface in stroke patients.
Data sources
The PubMed, Central, PEDro, Web of Science, SCOPUS, PsycINFO Ovid, CINAHL EBSCO, EMBASE, and ScienceDirect databases were searched from inception to April 2023 for randomized controlled studies reporting the effects of active transcranial direct current stimulation associated with brain–computer interface to a transcranial direct current stimulation sham associated with brain–computer interface condition on the outcome measure (motor performance and functional independence).
Review methods
We searched for full-text articles which had investigated the effect of transcranial direct current stimulation associated with brain–computer interface on motor performance in the upper extremities in stroke patients. The standardized mean differences derived from the change in scores between pretreatment and post-treatment were adopted as the effect size measure, with a 95% confidence interval. Possible sources of heterogeneity were analyzed by performing subgroup analyses in order to examine the moderating effects for one variable: the level of injury severity.
Results
Nine studies were included in the qualitative synthesis and the meta-analysis. The findings of the conducted analyses indicated there is not enough evidence to suggest that active transcranial direct current stimulation associated with brain–computer interface is more efficient in motor performance and functional independence when compared to sham transcranial direct current stimulation associated with brain–computer interface or brain–computer interface alone. In addition, the quality of evidence was rated very low. A subgroup analysis was performed for the motor performance outcome considering the injury severity level.
Conclusion
We found evidence that transcranial direct current stimulation associated with brain–computer interface was not more beneficial than sham transcranial direct current stimulation associated with brain–computer interface or brain–computer interface alone.
Get full access to this article
View all access and purchase options for this article.
References
1. Boden-Albala B, Appleton N, Schram B. Stroke epidemiology and prevention. In: Wilson R, Raghavan P (eds) Stroke rehabilitation. St. Louis, MO: Elsevier, 2019, pp.1–21.
2. Lee JJ. Lower limb impairments after stroke. In: Wilson R, Raghavan P (eds) Stroke rehabilitation. St. Louis, MO: Elsevier, 2019, pp.123–132.
3. Feng W, et al. Trans cranial direct current stimulation for poststroke motor recovery: challenges and opportunities. PM&R 2018; 10: S157–S164.
4. Elsner B, Kugler J, Pohl M, et al. Transcranial direct current stimulation (tDCS) for improving activities of daily living, and physical and cognitive functioning, in people after stroke. Cochrane Database Syst Rev 2016; 3: 1–168.
5. Fregni F, et al. Evidence-based guidelines and secondary meta-analysis for the use of transcranial direct current stimulation in neurological and psychiatric disorders. Int J Neuropsychopharmacol 2021; 24: 256–313.
6. Shih JJ, Krusienski DJ, Wolpaw JR. Brain-computer interfaces in medicine. Mayo Clin Proc 2012; 87: 268–279.
7. van Dokkum LEH, Ward T, Laffont I. Brain computer interfaces for neurorehabilitation—its current status as a rehabilitation strategy post-stroke. Ann Phys Rehabil Med 2015; 58: 3–8.
8. Danzl M, Chelette K, Lee K, et al. Brain stimulation paired with novel locomotor training with robotic gait orthosis in chronic stroke: a feasibility study. NeuroRehabilitation 2013; 33: 67–76.
9. Hesse S, et al. Combined transcranial direct current stimulation and robot-assisted arm training in subacute stroke patients: an exploratory, randomized multicenter trial. Neurorehabil Neural Repair 2011; 25: 838–846.
10. Cheng HJ, et al. Task-related brain functional network reconfigurations relate to motor recovery in chronic subcortical stroke. Sci Rep 2021; 11: 1–12.
11. Ang KK, et al. Facilitating effects of transcranial direct current stimulation on motor imagery brain-computer interface with robotic feedback for stroke rehabilitation. Arch Phys Med Rehabil 2015; 96: S79–S87.
12. Chew E, et al. Using transcranial direct current stimulation to augment the effect of motor imagery-assisted brain-computer interface training in chronic stroke patients—cortical reorganization considerations. Front Neurol 2020; 11: 948.
13. Hong X, et al. Brain plasticity following MI-BCI training combined with tDCS in a randomized trial in chronic subcortical stroke subjects: a preliminary study. Sci Rep 2017; 7: 9222.
14. Hu M, et al. Brain functional changes in stroke following rehabilitation using brain-computer interface-assisted motor imagery with and without tDCS: a pilot study. Front Hum Neurosci 2021; 15: 692304.
15. Mane R, et al. Prognostic and monitory EEG-biomarkers for BCI upper-limb stroke rehabilitation. IEEE Trans Neural Syst Rehabil Eng 2019; 27: 1654–1664.
16. Kasashima-Shindo Y, et al. Brain-computer interface training combined with transcranial direct current stimulation in patients with chronic severe hemiparesis: proof of concept study. J Rehabil Med 2015; 47: 318–324.
17. Straudi S, et al. tDCS and robotics on upper limb stroke rehabilitation: effect modification by stroke duration and type of stroke. Biomed Res Int 2016; 2016: 5068127.
18. Comino-Suárez N, et al. Transcranial direct current stimulation combined with robotic therapy for upper and lower limb function after stroke: a systematic review and meta-analysis of randomized control trials. J Neuroeng Rehabil 2021; 18: 148.
19. Guerra A, López-Alonso V, Cheeran B, et al. Solutions for managing variability in non-invasive brain stimulation studies. Neurosci Lett 2017; 719: 133332.
20. Guerra A, López-Alonso V, Cheeran B,. et al. Variability in non-invasive brain stimulation studies: reasons and results. Neurosci Lett 2020; 719: 133330.
21. Ang KK, Guan C. Brain-computer interface in stroke rehabilitation. J Comput Sci Eng 2013; 7: 139–146.
22. Bai Z, Fong KNK, Zhang JJ, et al. Immediate and long-term effects of BCI-based rehabilitation of the upper extremity after stroke: a systematic review and meta-analysis. J Neuroeng Rehabil 2020; 17: 1.
Source: https://journals.sagepub.com/doi/abs/10.1177/02692155231200086
[NEWS] Stroke rehab at home is near
Posted by Kostas Pantremenos in REHABILITATION, Tele/Home Rehabilitation on August 9, 2023
Date:August 8, 2023
Source:University of Houston
Summary:The world of at-home stroke rehabilitation is growing near, after the development of an EEG headset that connects the brain of stroke patients to powered exoskeletons for rehabilitation purposes.
FULL STORY
The world of at-home stroke rehabilitation is growing near, incredible news for the 795,000 people in the United States who annually suffer a stroke. A new low cost, portable brain-computer interface that connects the brain of stroke patients to powered exoskeletons for rehabilitation purposes has been validated and tested at the University of Houston.
“We designed and validated a wireless, easy-to-use, mobile, dry-electrode headset for scalp electroencephalography (EEG) recordings for closed-loop brain-computer (BCI) interface and internet-of-things (IoT) applications,” reports professor Jose Luis Contreras-Vidal, Hugh Roy and Lillie Cranz Cullen Distinguished Professor of electrical and computer engineering, in the journal Sensors. Contreras-Vidal is an international pioneer in noninvasive brain-machine interfaces and robotic device inventions.
An EEG-based brain-computer interface (BCI) is a system that provides a pathway between the brain and external devices by interpreting EEG. In other words, the device reads your mind, interpreting the brain’s activity to initiate robotic movement. Brain-machine interfaces based on scalp EEG also have the potential to promote cortical plasticity following stroke, which has been shown to improve motor recovery outcomes. The adjustable headset, designed from commercial off-the-shelf components, can accommodate 90% of the population. There is a patent-pending on both the BCI algorithm and the self-positioning dry electrode bracket allowed for vertical self-positioning while parting the user’s hair to ensure contact of the electrode with the scalp.
“We used a multi-pronged approach that balanced interoperability, cost, portability, usability, form factor, reliability and closed-loop operation,” said Contreras-Vidal.
In the current prototype, five EEG electrodes were incorporated in the electrode bracket spanning the sensorimotor cortices and three skin sensors were included to measure eye movement and blinks. An inertial movement unit, measuring head motion, allows for a portable brain-body imaging system for BCI applications.
“Most commercial EEG-based BCI systems are tethered to immobile processing hardware or require complex programming or set-up, making them difficult to deploy outside of the clinic or laboratory without technical assistance or extensive training. A portable and wireless BCI system is highly preferred so it can be used outside lab in clinical and non-clinical mobile applications at home, work, or play,” said Contreras-Vidal.
The invention solves an array of needs.
“Current commercial EEG amplifiers and BCI headsets are prohibitively expensive, lack interoperability, or fail to provide a high signal quality or closed-loop operation, which are vital for BCI applications,” said Contreras-Vidal.
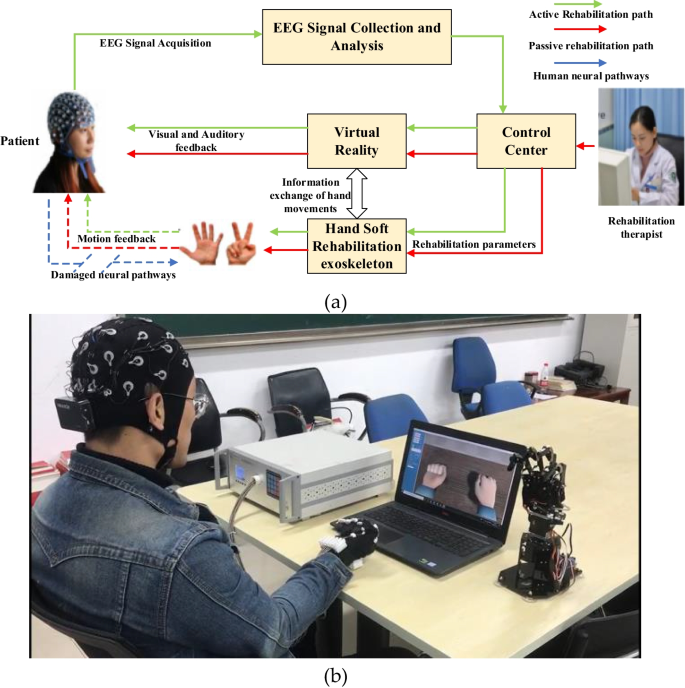
[ARTICLE] BCI–VR-Based Hand Soft Rehabilitation System with Its Applications in Hand Rehabilitation After Stroke – Full Text
Posted by Kostas Pantremenos in Paretic Hand, REHABILITATION, Virtual reality rehabilitation on June 13, 2023
Abstract
The recovery of hand motor function can effectively improve the living standard of stroke patients and relieve their psychological anxiety. Traditional physical rehabilitation training is unable to target the cause of motor function loss; therefore, the rehabilitation effect is not ideal. The objective of this study is to propose a hand rehabilitation system combining brain–computer interface (BCI), soft hand rehabilitation glove and virtual reality (VR), and explore its effectiveness on hand movement disorders in stroke patients. The corresponding comparison experiments conducted on 11 stroke patients demonstrated that the proposed BCI-based hand rehabilitation system can not only mobilize more cerebral cortex to participate in the process of hand motor rehabilitation, but also enhance the muscle strength, muscle tension, and improve the hand motor dysfunction of stroke patients.
1 Introduction
The number of elderly people suffering from vascular disorders has increased rapidly in developed countries. It has become a social problem, with consequences of paralysis and worsening living conditions. Many diseases can cause paralysis, for example, stroke, spinal cord injury (SCI), amyotrophic lateral sclerosis, and multiple sclerosis, in which stroke accounts for the largest proportion. According to the report of the World Health Organization (WHO) in 2016, stroke is now the second leading cause of death in the world. Most poststroke patients experience partial paralysis, which occurs mainly in the upper limbs, especially the hands [1, 2]. Ghassemi et al. [3] research has shown that six months after the onset of stroke, about 65% of stroke patients still have hand dysfunction, only 15% of stroke patients can recover about half of their hand function, and only 3% of patients are able to recover more than 70% of their original functions [4].
Stroke treatment for hand rehabilitation may require different equipment and methods, but physiotherapy (PT) is a central component of the rehabilitation process. Improving hand function requires repetitive task practice rehabilitation, which includes breaking tasks down into individual movements and practicing them to improve hand strength, accuracy, and range of motion. Constraint-induced motor therapy (CIMT), neuromuscular stimulation (NMS) and mental practice with motor imagery are some of the most common treatments for the rehabilitation of paraplegic hands after stroke, and their efficacy has been well established [5, 6]. However, these techniques have some important limitations, especially for patients in chronic stages. For example, nearly 50 percent of chronic patients with severe functional affectation do not experience improvement with CIMT, and residual motor activity is necessary for CIMT; therefore, CIMT is not suitable for stroke patients with severe limb weakness [7, 8].[…]