Posts Tagged Balance
[WEB] Revolutionizing Stroke Recovery: The iStride Device
Posted by Kostas Pantremenos in Assistive Technology, Gait Rehabilitation - Foot Drop, Rehabilitation robotics on January 19, 2024

The story of Maria Magdalena Valencia Juares, fondly known as Elena, is a testament to the power of innovation and the resilience of the human spirit. In 2021, Elena experienced a stroke that left her with muscle weakness and a decline in mental health, a situation further intensified by the isolation brought about by the COVID-19 pandemic. With her family residing thousands of miles away, they sought a solution to Elena’s predicament, and their answer came in the form of the iStride device. This ground-breaking tool, designed to help stroke survivors improve their walking ability, brought about incredible changes in Elena’s life, enabling her to climb stairs and dance again. The iStride device didn’t just offer her a solution; it provided hope and a renewed sense of independence.
The iStride Device: A New Hope for Stroke Survivors
The iStride device is a wearable technology designed to help stroke survivors regain their walking capability. Its real-time feedback mechanism supports users during walking exercises, leading to significant improvements in walking speed and endurance. As a result, stroke survivors gain better overall mobility and independence, as was the case with Elena.
Not only is the iStride device designed for functionality, but it is also built for comfort and adaptability. As a wearable ankle robot, it provides adjustable assistance to the user’s ankle, aiding in the regaining of strength and balance. Clinical studies corroborate the effectiveness of the iStride, with users reporting notable enhancements in their walking speed and endurance.
How the iStride Works
At its core, the iStride device is an assistive technology that aims to reduce the risk of falls in stroke survivors by providing targeted electrical stimulation to the muscles in the affected leg. This stimulation aids in improving strength and coordination, resulting in significant increases in walking speed and endurance, which in turn lead to greater independence and improved quality of life.
The iStride device employs a unique combination of visual and auditory cues to help patients improve their gait and balance. Its non-invasive nature and wearability ensure that it provides electrical stimulation to the leg muscles in a way that enhances mobility and minimises the risk of falls. The benefits of using the iStride device are not only immediate but also long-term, with studies showing sustained improvements in walking ability for stroke survivors, even one year after therapy.
iStride: Shifting the Paradigm of Stroke Recovery
The remarkable story of Elena serves as an inspiring testament to the potential of the iStride device. It is not just a rehabilitation tool; it is a device that promises a shift in the paradigm of stroke recovery. The iStride device offers a beacon of hope to stroke survivors, enabling them to regain their independence and significantly improve their quality of life.
As we move forward, it is clear that the iStride device will continue to empower stroke survivors, offering them a chance to reclaim their mobility, independence, and ultimately, their lives. Through technology and innovation, we can look forward to a future where stroke recovery is not just possible, but also achievable and sustainable.
[ARTICLE] Mild Stroke, Serious Problems: Limitations in Balance and Gait Capacity and the Impact on Fall Rate, and Physical Activity – Full Text
Posted by Kostas Pantremenos in Gait Rehabilitation - Foot Drop on November 9, 2023
Abstract
Background
After mild stroke persistent balance limitations may occur, creating a risk factor for fear of falling, falls, and reduced activity levels. Objective. To investigate whether individuals in the chronic phase after mild stroke show balance and gait limitations, elevated fall risk, reduced balance confidence, and physical activity levels compared to healthy controls.
Methods
An observational case-control study was performed. Main outcomes included the Mini-Balance Evaluation Systems Test (mini-BEST), Timed Up and Go (TUG), 10-m Walking Test (10-MWT), and 6-item version Activity-specific Balance Confidence (6-ABC) scale which were measured in 1 session. Objectively measured daily physical activity was measured for 7 consecutive days. Fall rate in daily life was recorded for 12 months. Individuals after a mild stroke were considered eligible when they: (1) sustained a transient ischemic attack or stroke longer than 6 months ago, resulting in motor and/or sensory loss in the contralesional leg at the time of stroke, (2) showed (near-) complete motor function, that is, ≥24 points on the Fugl-Meyer Assessment—Lower Extremity (range: 0-28).
Results
Forty-seven healthy controls and 70 participants after mild stroke were included. Participants with stroke fell more than twice as often as healthy controls, had a 2 point lower median score on the mini-BEST, were 1.7 second slower on TUG, 0.6 km/h slower on the 10-MWT, and had a 12% lower 6-ABC score. Intensity for both total activity (8%) as well as walking activity (6%) was lower in the participants with stroke, while no differences were found in terms of duration.
Conclusions
Individuals in the chronic phase after a mild stroke demonstrate persistent balance limitations and have an increased fall risk. Our results point at an unmet clinical need in this population.
Introduction
Balance and gait limitations, resulting from impaired sensorimotor function, are an important contributor to disability and reduced quality of life post-stroke.1–3 Rehabilitation programs, therefore, often aim to improve balance and gait performance in individuals with profound sensorimotor impairments after stroke.4 However, in a substantial proportion of patients with initial sensorimotor impairments, these impairments recover relatively quickly after stroke, ultimately resulting in (near-) absent sensorimotor symptoms.5 Those individuals with a so-called “mild stroke” are often discharged home after a short hospital stay,6,7 yet they face challenges to resume their daily life activities after hospital discharge.7 These challenges in daily life might be related to persistent balance and gait limitations. Given the paucity of studies and practice guidelines regarding persisting motor impairments in individuals after mild stroke, more insights are required into the presence and potential consequences of balance and gait limitations in this specific group.
Balance and gait limitations after stroke are a key risk factor for accidental falls.8 Falling is a major problem in moderately to severely affected individuals after stroke, as their fall risk remains substantially elevated (2-10 fold increase) throughout all post-stroke phases as compared to the general population of older adults.8 Whether fall risk is also elevated in people with minor stroke has not yet been studied. This deserves further investigation, since falls can have serious physical and psychological consequences. Direct physical consequences of falls involve bruises, soft tissue injuries, and also more severe injuries such as fractures (0.6%-15% of the post-stroke falls).8,9 Moreover, falls after stroke have a substantial psychological impact, with 88% of individuals after stroke developing a subsequent fear of falling after the incident.10,11 Individuals after stroke who fell tend to become less physically active11 either due to fear of falling12 or because of physical injuries. In the long term, avoidance of activities contributes to restricted social participation as well as reduced cardiovascular fitness. As physical activity levels in community-dwelling individuals in the chronic phase after stroke are already low in terms of duration and intensity,13 further deconditioning may contribute to loss of independence. In sum, post-stroke balance and gait limitations and subsequent (risk of) falls have serious physical and psychological consequences that eventually result in limited physical activity. Fortunately, exercise appears to reduce fall rates after stroke,14 and well-designed exercise therapy is effective in reducing balance limitations in the chronic phase after stroke.15
While the literature on individuals with mild motor impairments after stroke is sparse, a few studies report impaired balance and gait capacity after mild stroke.16–19 However, it remains unclear what the impact of these balance and gait limitations is in terms of falls and their consequences. We therefore conducted an observational case–control study of individuals in the chronic phase after mild stroke in which balance and gait capacity, balance confidence, and physical activity were measured along with a 12-month follow-up period of self-reported falls. In this study, we addressed the following research questions: What is the fall rate in individuals in the chronic phase after mild stroke as compared to healthy individuals? Do individuals with mild stroke present with limitations in terms of balance and gait capacity and are they less physically active as compared to healthy individuals? And if so, do these differences also pertain to individuals after mild stroke without clinically-established lower limb impairments (ie, maximum Fugl-Meyer and Motricity Index scores)?
We hypothesized that, compared to healthy individuals, balance and gait capacity would be reduced in individuals in the chronic phase after mild stroke as measured with the mini-Balance Evaluation Systems Test (mini-BEST, Timed up and go [TUG], and 10-m walking test [10-MWT]). Furthermore, we hypothesized that participants with mild stroke would exhibit higher fall rates, greater fear of falling (as measured with the Activity-specific Balance Confidence [ABC] scale), as well as lower physical activity levels (as measured with a wearable activity monitor) than their healthy counterparts. […]

[BLOG POST] All You Should Know About Brain Stroke
Posted by Kostas Pantremenos in Educational on March 23, 2023
All You Should Know About Brain Stroke
A stroke, sometimes called a brain attack, occurs when blood vessel supplying ay part of brain gets blocked or bursts. In either case, parts of the brain become damaged or die. A stroke can cause lasting brain damage, long-term disability, or even death. Brain stroke is 3rd most common killer disease in the world.
What are the types of strokes?
Ischemic stroke: It occurs when blood vessel gets blocked leading to death of part of brain supplied by the blood vessel.
Hemorrhagic stroke: It occurs when blood vessel bursts leading to bleeding within brain tissue and damage of tissue.
One more entity called transient ischemic attack (TIA), as the name suggests stroke symptoms start and resolve on own due t restoration of blood supply to brain. Many times TIAs occur days or weeks before actual stroke, hence acting as warning sign for future stroke. Recognizing and treating TIAs can avert major strokes.
What happens during stroke:
Brain needs continues supply of oxygen and nutrients. Due to blocked or burst blood vessel energy supply gets affected leading to death of neurons within minutes. Each half of brain roughly controls opposite half of body. Right Brain stroke leads to problems in left half of body and vice versa.
What are the risk factors for stroke?
Risk factors for stroke can be classified as modifiable (that can be changed) and non-modifiable(that can’t be changed).
Modifiable risk factors: High blood pressure( > 140/90), Diabetes mellitus, Lack of sleep, Stress, Obesity, Smoking, High plasma lipids, Lack of exercise, Oral contraceptive pills, heart disease, abnormal heart rhythm.
Non modifiable risk factors are: older age, male gender, ethnicity- African Americans, history of prior strokes, genetic risk factors for strokes.
What are the symptoms of stroke:
Stroke symptoms happen suddenly, symptoms vary and can occur in combination of any of the following:
- Weakness or numbness of the face, arm, or leg, usually on one side of the body
- Having trouble speaking or understanding
- Problems with vision, such as dimness or loss of vision in one or both eyes
- Dizziness or problems with balance or coordination, vomiting
- Problems with movement or walking
- Fainting (loss of consciousness) or seizure
- Severe headaches with no known cause, especially if they happen suddenly
Stroke symptoms can be easily identified with acronym BEFAST: Balance, Eye problems, Facial droop, Arm weakness, Speech change, Time since onset of symptoms. If any of these symptoms occur, time of onset should be noted and immediate medical attention should be sought.
Treatment of stroke: Treatment is immediate evaluation, medical, surgical, rehabilitative.
As soon as Stroke is suspected neuroimaging in the form of CT or MRI brain is needed. Type of stroke need to be identified- Ischemic (due to blocked blood vessel) or hemorrhagic (burst blood vessel)
Medical: If patient reaches hospital within 4.5hrs of symptoms onset, Injection called tissue plasminogen activator can be given in eligible patients to dissolve clot and restore blood supply. This is used only in ischemic stroke.
Surgical or interventional: If a large blood vessel is blocked, a procedure called mechanical thrombectomy is done where in clot is removed with endovascular techniques to restore blood vessel. This can be done up to 24hr from symptom onset in eligible patients.
Rehabilitation: patients who have persistent neurological deficits need rehabilitation in the form of physiotherapy, balance therapy, speech therapy. Rehabilitation can take months in major strokes.
Stroke can strike anyone, anytime and can change one’s life forever. Prevention with risk factor modification, good lifestyle, early detection of warning signs, seeking medical attention on time can prevent major disability and death.
[Abstract] Virtual reality for balance and mobility rehabilitation following traumatic brain injury: A systematic review of randomized controlled trials
Posted by Kostas Pantremenos in REHABILITATION, TBI, Virtual reality rehabilitation on March 17, 2023
Abstract
Background: Balance and mobility deficits are most prevalent impairments in patients with traumatic brain injury (TBI). The evidence has proposed that rehabilitation plays an important role in improving balance and mobility post-TBI. Virtual reality (VR) is a computer technology that provides immersed users to generate feedback such as visual, audio, and haptic.
Objective: This review aimed to examine the effects of the VR treatment intervention on balance and mobility in patients with TBI and to define the most effective VR treatment protocol.
Methods: SCOPUS, PEDro, PubMed, REHABDATA, EMBASE, and the web of science were searched for experimental trials examining the impacts of VR training on balance and mobility in patients with TBI from inception until July 2022. Physiotherapy Evidence Database (PEDro) scale was used to assess the methodological quality of the selected studies.
Results: Five randomized controlled trials (RCTs) met the inclusion criteria. The PEDro scores ranged from 6 to 8, with a median of 6. A total of 157 patients with TBI were included in this review, 31.2% of whom were females. The findings showed that VR intervention is not superior to traditional physiotherapy interventions in improving balance and mobility post- TBI.
Conclusions: The preliminary findings showed that the influence of VR on the balance and mobility ability in patients with TBI is promising. Combining VR with other concurrent rehabilitation interventions may show more significant improvements in balance and mobility compared to VR interventions alone. The optimal VR treatment protocol remains unclear. Further randomized controlled trials are strongly needed.
[WEB] Dual Task in Gait Rehabilitation
Posted by Kostas Pantremenos in Gait Rehabilitation - Foot Drop on January 24, 2023

Advanced technology allows rehab professionals to better implement dual task science to improve a patient’s gait. Can you afford to practice without it?
By Mike Studer, PT, DPT, MHS, NCS, CEEAA, CWT, CSST, FAPTA
We know that our worlds are busy, and for many, increasingly busier. Are our sessions in rehabilitation representing a busy world well enough? Are we challenging our patients to be able to tolerate and thrive or even just sustain safe mobility when distracted? In this article we will explore the benefits of applying dual-task (DT) walking for your patients with impaired gait and dynamic balance, with an acknowledgement of some of the ancillary benefits of DT along the way. Recent advances in technology are proving promising and may be a perfect fit for your patients, and your clinic right now.
Multi Diagnostic Fall Risk and Measure of Performance
Fall risk in the elderly is the most prolifically studied group across articles of dual task. Many conditions outside of aging have been studied with great promise as well. In no specific order, these include concussion, stroke, Parkinson Disease, Multiple Sclerosis, and neuropathy. It should be noted that dual task science is also routinely studied in the sport performance world within the context of promoting skill acquisition and even automaticity. Recently, the consideration of tolerating a secondary distractor has also been leveraged in military exercises, training the tactical warrior.
The science of dual tasking includes examination (DT tolerance) and treatment (using DT interference, or DTI). Technological advances are making it more intuitive for the therapist and patient alike to capture objective data about DT tolerance, which is often expressed as a percentage of performance without distraction, or DT Cost (DTC). Within the realms of DT examination, the science supports use of dual task in prescriptive manner, using DTC as a prodromal diagnostic tool (early detector of PD and AD) as well as a prognostic tool or clinical indicator in the case of those returning to prior function after injury, most notably sport-related concussion.
Podcast: Listen to Mike Studer talk to Rehab Management about Dual Task in Return to Sport After Concussion
Similarly and increasingly, DTI has been used in rehabilitation as an outcome measure for persons with stroke (PwS), concussion, imbalance/fall risk, and in those with a known or suspected degenerative disease. In this manner, clinicians increasingly use the DTC equation to express impairment and improvement in a functional task with superimposed distraction.
Science of Dual Task
The science of dual task is continuing to evolve and improve in both examination and intervention. As with any measure, the reliability and validity, predictive value, and methodology of dual task paradigms are being considered across hundreds of research articles per year. Interventions that use DTI are improving as well. Specifically, the science of DT is increasingly more practical, task specific, and more precise in dosage, bringing more variety and making the treatments more engaging for patients, while applications become more intuitive for therapists. Technology for dual task testing and training in gait is now viable for the most severely impaired (using locomotor robotics and VR) and the most high-level patients (using overground walking and challenging cognitive tasks). DT technologies are among the most compelling improvements that the rehabilitation professional can offer to their patients today, utilizing gamification, virtual reality, and wearables that can capture and objectify movement performance through more person-specific contexts.
Therapists can additionally utilize DT science coupled with technology in skilled care documentation. Utilizing objective data such as the actual sway recorded in centimeters while both distracted and focused, each conditions of a modified Clinical Test of Sensory Interaction on Balance (mCTSIB), remains more valuable to reviewers than a qualitative scoring system. Expressing fall risk by interlimb variability recorded gait comparing focused single task to walking-with-a-working-memory task remains superior to a physical-only measure of distance/time. Documenting DT cost in a 30-second sit-to-stand captured with and without distraction is more sophisticated than a raw 30SSTS score alone.
While these are irrefutable examples of combining the science of DT with technology, even these do not tell the whole story of the clinical value. Consider the ceiling effect that limits our physical-only and cognitive-only measures as we advocate for patients to realize their full potential and community re-integration. Dual task expands the conversations, and potentially the authorization.
Engaging Technological Advances for Patient and Therapist
Some of the most compelling technological advances in DT have come into existence within the last two years. It is now within reach for therapists to affordably and conveniently adapt their current treadmill with or without body weight support to become both a gait laboratory and an engaging space for their patients. On most platforms, therapists and patients alike benefit from body worn sensors, instrumented treadmills, or camera recordings to collect outcome data about physical performance. All of the gait variables that you want, and some that you may not be able to track as easily with your eyes, are available.
Can you see yourself conducting a Timed Up and Go as a shuttle test (several laps) with a person with Parkinson Disease while the patient interacts with a computerized display that requires working memory? Can your athlete recovering from sport-related concussion benefit from objective measures that help her see and hear that her balance under dual task conditions is back to the baseline measurement that she displayed in preseason screening? Technology measures the performance, potentially making this more valid and objective in the mind of the patient. Finally, imagine the excitement of your 9-year-old patient with cerebral palsy who is excited about following the sequence of colors in the same order that it was presented on the board.
As Anne Shumway-Cook and Marjorie Woollacott reference in their newest edition of Motor Control, therapists may realize the benefits of these technologies as we give greater consideration for and actualized potential within the Task-Environment-Individual interaction. In these cases, technology can support the principles of motor learning through skilled therapy. The compelling nature of DT can permit one to participate longer (endurance and repetitions), engage more deeply (attention), or compete for a new high score (intensity). Clearly, the combination of technology with engaging games or environments can elevate the efficacy of our therapeutic environments.
The technology of dual task for gait and balance can make for some of the most widely attended and popular booths at larger meetings and conventions, including the American Physical Therapy Association’s (APTA) upcoming Combined Sections Meeting in San Diego. These technological advances make the exposition center a must, and a value-added part of the conference experience. If you have not yet had the experience to be engaged in dual task technology technological applications in gait, a conference exposition center is a great place to experience the advances firsthand. Until then, the accompanying photos and resource box may be of assistance for your vicarious experience and due diligence.
Finally, the next time you find yourself walking through your hometown to a local grocery store while remembering your grocery list and avoiding those puddles along the way…just know that your patients want to experience that again, and that you should prefer that they have it within your care before facing it alone.
Mike Studer, PT, DPT, MHS, NCS, CEEAA, CWT, CSST, FAPTA, is a co-owner of Spark Rehabilitation and Wellness in Bend, Ore. He is also an adjunct professor at Oregon State University’s DPT program, where he leads the coursework on motor control. Studer is a world-recognized educational provider and clinician in the areas of stroke, functional neurologic disorders, Parkinson Disease, and more. For more information, contact RehabEditor@medqor.com.
[WEB] Young Stroke Program: Path to Get Back into Action
Posted by Kostas Pantremenos in REHABILITATION on December 21, 2022

by Stephanie Zanvettor, PT, CBIS, CCI
Every year, nearly 800,000 Americans are faced with the challenge of recovery after a stroke.1 While the majority of strokes occur in people aged 65 and older, the incidence has been increasing in adults aged 49 years and younger over the last three decades. This can potentially be attributed to the fact that risk factors for stroke such as high blood pressure and type 2 diabetes have been on the rise among younger and middle-aged adults.2 In 2014, nearly 40% of people hospitalized for stroke were less than 65 years old.1
With a keen understanding that recovery and rehabilitation is not a one-size-fits-all solution for those who led an active life before their stroke, the medical and rehabilitation staff at Gaylord Specialty Healthcare—a Connecticut rehabilitation-focused healthcare system with CARF specialty accreditation for its stroke program—launched the Young Stroke Program to give this generally younger and more active segment of stroke patients the customized, rigorous care needed to help them return to baseline.
Young Stroke: A Program for All Ages
As therapists and clinicians, we know that the impact of a stroke extends well beyond the survivor’s physical deficits. Patients can be deeply impacted emotionally, socially, and financially, and the impact often ripples out to affect family, friends, and colleagues. The toll can be even more devastating if the stroke survivor was a significant financial contributor in their household, provided care for a young child or family member, or was pursuing education.
In 2010, Gaylord established its Young Stroke program, a segment of its inpatient stroke program, with the specific goal of preparing survivors who led active lives at the time of their stroke to return to as close as possible to their baseline level of activity through aggressive rehabilitation and a highly individualized, goal-driven plan of care.
The program’s name, “Young Stroke,” can be deceiving. Although more than 45% of Gaylord’s inpatient stroke population is 65 years old or younger, the primary qualification for the Young Stroke Program is not based on age. Rather, eligibility is directly related to the patient’s level of activity prior to their stroke, particularly if they were:
- Significantly and actively employed
- A student, or,
- A primary caregiver
Those with goals to return to any of these functions post-stroke—regardless of age—may be deemed good candidates for the program.
A Different Approach to Stroke Rehabilitation
The Young Stroke program takes advantage of the overall greater levels of energy and resiliency of this patient population. Stroke survivors who were very active prior to their stroke are often ready, willing, and able to participate in a more focused, rigorous program to help them return to the life they led pre-stroke.
Last year, 38% of Gaylord Specialty Healthcare’s inpatient stroke population participated in the “Young Stroke” program, their ages ranging from 15 to 82 years old, with the average age of 55. In comparison, the average age of stroke patients who did not fall into the Young Stroke program was 72.6, with ages ranging from 30 to 96.
The Young Stroke patient’s journey begins in the inpatient setting with a strong, interdisciplinary care team consisting of medical, nursing, therapy, care management, psychology, nutrition, respiratory, and chaplain services. Goal setting begins on day one and is considered the nucleus of the patient’s plan of care.
Most survivors participate in three hours of therapy per day or more, consisting of one-on-one and skilled group sessions with activities to promote fine motor coordination, cognitive skills, balance, and mobility. Survivors can also participate in activities with therapeutic recreation including cooking skills, virtual reality, visits with the therapy dog, and yoga. Survivors also participate in a stroke education series to learn about the anatomy of their stroke, recovery challenges, and advocacy.
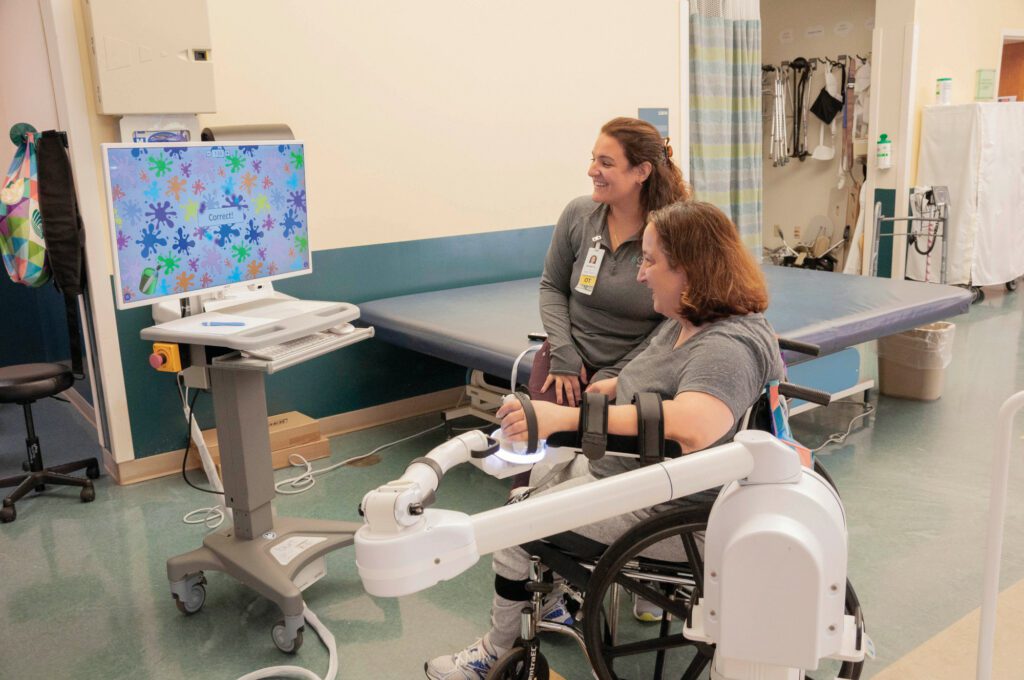
Photo: Karen Ingham Photography
Skills to Return to Former Activities
In addition to participating in an aggressive physical, occupational, and speech therapy program, Young Stroke patients work on regaining or improving the particular skills they need to achieve their goals of returning to school, work, or caregiving.
When working with students in the Young Stroke program, speech and occupational therapists may be involved in finding adaptive techniques or equipment to help them take notes or exams. Therapists may also make recommendations to the school to ensure the student’s long-term success.
A survivor who is a primary caregiver may engage in activities related to caring for a child such as picking up an infant or toddler, feeding, diapering, bathing, dressing, or transferring the child to a car seat, crib, or stroller. To facilitate the transition to home, family training with the patient’s significant other is encouraged to help in sharing the care of the child and building the survivor’s confidence in handling skills required at home.
A survivor who was previously employed may practice his or her typing skills on a computer or work on tasks such as spreadsheets. If handwriting or the use of hand-held tools is central to performing a patient’s specific job, a therapist may work on grip strength and upper extremity strength with the help of robotics and electrical stimulation. Young Stroke patients also have access to a number of other technologies including a body-weight-supported gait and balance system to safely challenge a patient’s balance while walking. Consultations with a neuro optometrist to address any potential vision or attention deficits are also often incorporated into a patient’s plan of care.
Many survivors express their desire to return to driving. Before discharge, they are offered education on available return-to-driving programs and timeline expectations. When appropriate, they may participate in a brake reaction test to evaluate reaction time as one component of the gross motor skill of driving.
Young Stroke Program Discharge Plans
As their journey continues, the survivor will participate in discharge planning as the team works with them and their families to create a safe discharge plan. Last year, nearly 62% of Gaylord’s Young Stroke patients were discharged to home, either with or without home care services. In comparison, 41% of stroke patients who do not fall into the Young Stroke category were discharged to home. Many patients in both programs continue their recoveries with outpatient neurorehabilitation services.
A small segment (fewer than 5%) of the Young Stroke inpatient population is discharged directly to Gaylord’s on-campus Traurig House, the state’s only transitional living home for people recovering from brain injury and stroke. The residential program offers a home-like setting with programs to support daily living skills and access to the Gaylord outpatient rehabilitation they need to further regain the skills and confidence to successfully return to their homes and communities.
Case Study: College Professor
Andy*, a professor in his early 40s and father to young children, had a stroke that left him with severe speech and short-term memory deficits. Andy had two goals: to return to caring for his children and to return to the classroom the next semester.
After his inpatient stay, Andy was discharged to live in Gaylord’s Traurig House and continued with outpatient therapy through the hospital’s Day Treatment Program.
In addition to rigorous physical and occupational therapies, Andy worked closely with a speech therapist to help him meet his lofty goal of returning to teaching only six months later.
In an effort to make Andy’s therapy as realistic as possible, the speech therapist encouraged him to revise his syllabi for the upcoming semester to help him work on fine-tuning his language and memory strategies. The therapist also encouraged Andy to arrange and present a mini-lecture to scores of Gaylord employees during their lunch hour. For weeks, he worked on preparing a lecture to fit the allotted time, creating a visual presentation, and practicing his verbal skills.
The rigorous preparation and shot of confidence that his preparation and subsequent successful presentation gave Andy enabled him to fulfill his goal of returning the following semester and, most importantly, be active with his kids once again.
Case Study: Working Grandmother
At the age of 55, Dara* was employed full time and was actively involved in the life of her grandchild when she suffered a stroke in the right posterior basal ganglia region of the brain. Her goals were to go home, regain the use of her left side for an eventual return to work, and help out in the care of her grandchild.
She initially presented with a flaccid left side and worked tirelessly in her therapies to regain her independence. Dara was able to take advantage of many of Gaylord’s technologies to assist in her recovery, including an e-stim device for her upper extremity to assist with hand function and lower extremity to assist with foot drop, an overhead body-weight support system to challenge her balance and gait, and a bionic exoskeleton to gain endurance and confidence with weight bearing.
Her initial falls risk as measured by the BERG balance scale was a 6, indicating a high falls risk. At discharge, Dara scored a 42 on the BERG, indicating a low falls risk.
She initially required 75% assist of caregiver for upper body dressing, 100% assist for lower body dressing, and 25% assist for toileting. Upon discharge directly to home, Dara was independent for toileting and dressing and required supervision for showering.
Dara’s initial Fugl Meyer Score (a measure of upper extremity function) was a 16 for her left upper extremity and upon discharge she scored a 25/66 for her left upper extremity, indicating that she had gained mobility in her shoulder, elbow, wrist, and hand function. Within an inpatient length of stay of three weeks, Dara went from a 25% assist for mobility to supervision to modified independent level at home with continued therapy.
Dara was recently discharged home with the recommendation of continued therapy through Gaylord outpatient.
Both case studies are excellent examples of how Gaylord’s Young Stroke program drives exceptional outcomes.
In conclusion, survivors of any age who led active lives at the time of their stroke can greatly benefit from such an inpatient program to prepare them to return as close as possible to their baseline level of activity through aggressive rehabilitation and a highly individualized, goal-driven, and interdisciplinary plan of care.
*Name changed.
Stephanie Zanvettor, PT, CBIS, CCI is an inpatient physical therapist for Gaylord Specialty Healthcare in Wallingford, Conn. She received her master’s degree in physical therapy at Ohio University and currently specializes in neurological rehabilitation and has a passion for working with patients after stroke and traumatic brain injuries.
Main Photo Caption: Stroke survivors who were very active prior to their stroke are often ready, willing, and able to participate in a more focused, rigorous program to help them return to the life they led pre-stroke. Here, a patient practices cooking skills. Photo: Karen Ingham Photography
References
1. Stroke Facts. Centers for Disease Control and Prevention. https://www.cdc.gov/stroke/facts.htm
2. U.S. Stroke Rate Declining in Adults 75 and Older, Yet Rising in Adults 49 and Younger. The American Stroke Association. Feb. 3, 2022. https://newsroom.heart.org/news/u-s-stroke-rate-declining-in-adults-75-and-older-yet-rising-in-adults-49-and-younger
[ARTICLE] Virtual Reality Training Using Nintendo Wii Games for Patients With Stroke: Randomized Controlled Trial – Full Text
Posted by Kostas Pantremenos in Paretic Hand, Video Games/Exergames, Virtual reality rehabilitation on December 12, 2022
Abstract
Background
Stroke is a leading cause of disability. It is difficult to devise an optimal rehabilitation plan once stroke survivors are back home. Conventional rehabilitative therapies are extensively used in patients with stroke to recover motor functioning and disability, but these are arduous and expensive. Virtual reality (VR) video games inspire patients to get involved in their therapeutic exercise routine in a fun way. VR in the form of games provides a fruitful, secure, and challenging learning environment for motor control and neural plasticity development in rehabilitation. The effects of upper limb sensorimotor functioning and balance are the main focus of this trial.
Objective
The aim of this study is to compare the effects of VR training and routine physical therapy on balance and upper extremity sensorimotor function in patients with stroke.
Methods
It was a single assessor-blinded randomized clinical trial. A total of 74 participants with their first chronic stroke were included and rehabilitated in a clinical setting. The lottery method was used to randomly assign patients to either the VR group (n=37) or the routine physical therapy group (n=37). The VR group received a 1-hour session of VR training for 3 weekdays over 6 weeks, and the routine physical therapy group received different stretching and strengthening exercises. The outcome measuring tools were the Berg Balance Scale for balance and the Fugl-Meyer Assessment (upper extremity) scale for sensorimotor, joint pain, and range assessment. The assessment was done at the start of treatment and after the 6 weeks of intervention. Data analysis was done using SPSS 22.
Results
The trial was completed by 68 patients. A significant difference between the two groups was found in the Berg Balance Scale score (P<.001), Fugl-Meyer Assessment for motor function (P=.03), and Fugl-Meyer Assessment for joint pain and joint range (P<.001); however, no significant difference (P=.19) in the Fugl-Meyer Assessment for upper extremity sensation was noted.
Conclusions
VR training is helpful for improving balance and function of the upper extremities in the routine life of patients with stroke; although, it was not found to be better than conventional training in improving upper limb sensation. VR training can be a better option in a rehabilitation plan designed to increase functional capability.
Introduction
Stroke is a leading cause of disability, and it is difficult to devise an optimal rehabilitation plan for patients with stroke once they are discharged from the hospital [1]. Almost 85% of patients with stroke have hemiparesis after stroke while 55% to 75% of stroke survivors have motor dysfunction. South Asian people (people of India, Pakistan, Sri Lanka, Bangladesh, Nepal, and Bhutan) have a higher risk of stroke because of compromised cardiac and metabolic rate [2,3]. Treatment for stroke is initiated with drugs [4], and surgery might be another option to repair any constriction or narrowing of blood vessels [5,6]. Patient rehabilitation is an important part of treatment. The main purpose of rehabilitation is to improve the quality of life for patients with stroke and make them independent [7,8]. Conventional rehabilitative therapies are extensively used to help patients with stroke recover motor functioning and disability. However, the application of conventional techniques is arduous and expensive, and requires transportation of patients to tertiary care hospitals especially in countries like Pakistan where hospitals are less in number. Virtual reality (VR) training in the form of games [9] provides a fruitful, secure, and challenging learning environment for motor control and neural plasticity development after stroke. VR video games inspire patients to get involved in their therapeutic exercise routine in a fun way [10]. Depending on the remodeling and reorganization of brain function, previous studies found that VR can be a great alternative for quick functional recovery after stroke [11]. Mirror neurons in the cortex can be activated through observational learning by VR training. Participants who received sensory input in VR training were also more likely to learn the desired motor behavior [12]. The feedback can help to promote the development of use-dependent cortical plasticity, which could lead to improved motor control. Furthermore, the functional improvement induced by VR training could significantly boost participants’ confidence and self-efficacy in a new environment. Moreover, another advantage of VR is that it can greatly save on labor and cost of patients [13].
Stroke is seen to be more prevalent in countries like Pakistan, as the people are more inclined toward using local drugs like naswar, pipe smoking, and beetle leaf chewing (paan). Thus, there is higher incidence of stroke in middle-aged populations (<45 years) [14–16]. The study aimed for a younger population with stroke and found cost-effective treatment protocols at the same time. The unique needs of young people with stroke and the promising opportunity provided by a low-cost serious game would be a beneficial addition in treatment strategy. There is inadequate evidence in the literature to generalize effects on upper limb sensorimotor function and gait through commercial gaming in young patients with stroke. Studies on effectiveness of VR programs in comparison to traditional methods on functional-motor improvement of an upper limb are needed in low-resource countries to reduce cost and time through target-oriented interventions. This study was conducted to compare the effects of VR training and routine physical therapy on balance and function of upper limbs in patients with stroke from the lower- and middle-class populations. This study is conducted to accept or reject the hypothesis that VR has a significantly better effect on balance and upper limb function in patients with stroke.[…]
[BLOG POST] The VR Device For ALL Your Rehab Needs.
Posted by Kostas Pantremenos in REHABILITATION, Virtual reality rehabilitation on November 26, 2022


-Shoulder & Elbow Function
-Grip & Release Strengthening
-Gross Muscle Strengthening
-Upper Extremity Robotic Compatible
-Functional Reaching

-Hip, Knee, & Ankle Function
-Gross Muscle Strengthening
-Bike, Nu-Step, & Standing Frame Compatible
-Weight Bearing & Weight Shifting

-Cervical ROM
-Cervical & Trunk Proprioception
-Vestibular Rehab
-Posture
-Functional Balance & Stability
-Weight Shifting & Weight Bearing

-Chronic/Acute Pain
-Distraction
-Controlled Breathing
-Decompression
-Anxiety Alleviation

-Audio Que Recognition
-Visual-Spatial Awareness
-Attention
-Money Management
-Listening Skills

-Functional Reaching
-Functional Balance and Stability
-Grocery Shopping
-Money Management
[Abstract] Development of a convolutional neural network (CNN) based assessment exercise recommendation system for individuals with chronic stroke: a feasibility study
Posted by Kostas Pantremenos in REHABILITATION on November 7, 2022
ABSTRACT
Background
The use of artificial intelligence (AI) is revolutionizing nearly every aspect of healthcare, but the application of AI in rehabilitation is lagging behind. Clinically, gait parameters and patterns are used to evaluate stroke-specific impairment. We hypothesized that gait kinematics of individuals with stroke provide rich information for the deep-learning to predict the clinical decisions made by physiotherapist.
Objective
To investigate whether the results of clinical assessments and exercise recommendations by physiotherapists can be accurately predicted using a deep-learning algorithm with gait kinematics data.
Method
In this cross-sectional study, 40 individuals with stroke were assessed by a physiotherapist using the lower-extremity subscale of the Fugl-Meyer Assessment (FMA-LE) and Berg Balance Scale (BBS). The physiotherapist also decided whether or not the single-leg-stance was an appropriate balance training for each participant. The participants were classified as having good mobility and a low fall risk based on the cutoff scores of the two clinical scales. A convolutional neural network (CNN) was trained using gait kinematics to predict the assessment results and exercise recommendations.
Results
The trained model accurately predicted the results of the clinical assessments and decisions with an average prediction accuracy of 0.84 for the FMA-LE, 0.66 for the BBS, and 0.78 for the recommendation of the single-leg-stance exercise.
Conclusions
This CNN deep-learning model provided time-effective and accurate prediction of clinical assessment results and exercise recommendations. This study provides preliminary evidence to support the use of biomechanical data and AI to assist treatment planning and shorten the decision-making process in rehabilitation.

