Posts Tagged motor control
[ARTICLE] Human-machine-human interaction in motor control and rehabilitation: a review – Full Text
Posted by Kostas Pantremenos in REHABILITATION on September 25, 2022
Abstract
Background
Human-human (HH) interaction mediated by machines (e.g., robots or passive sensorized devices), which we call human-machine-human (HMH) interaction, has been studied with increasing interest in the last decade. The use of machines allows the implementation of different forms of audiovisual and/or physical interaction in dyadic tasks. HMH interaction between two partners can improve the dyad’s ability to accomplish a joint motor task (task performance) beyond either partner’s ability to perform the task solo. It can also be used to more efficiently train an individual to improve their solo task performance (individual motor learning). We review recent research on the impact of HMH interaction on task performance and individual motor learning in the context of motor control and rehabilitation, and we propose future research directions in this area.
Methods
A systematic search was performed on the Scopus, IEEE Xplore, and PubMed databases. The search query was designed to find studies that involve HMH interaction in motor control and rehabilitation settings. Studies that do not investigate the effect of changing the interaction conditions were filtered out. Thirty-one studies met our inclusion criteria and were used in the qualitative synthesis.
Results
Studies are analyzed based on their results related to the effects of interaction type (e.g., audiovisual communication and/or physical interaction), interaction mode (collaborative, cooperative, co-active, and competitive), and partner characteristics. Visuo-physical interaction generally results in better dyadic task performance than visual interaction alone. In cases where the physical interaction between humans is described by a spring, there are conflicting results as to the effect of the stiffness of the spring. In terms of partner characteristics, having a more skilled partner improves dyadic task performance more than having a less skilled partner. However, conflicting results were observed in terms of individual motor learning.
Conclusions
Although it is difficult to draw clear conclusions as to which interaction type, mode, or partner characteristic may lead to optimal task performance or individual motor learning, these results show the possibility for improved outcomes through HMH interaction. Future work that focuses on selecting the optimal personalized interaction conditions and exploring their impact on rehabilitation settings may facilitate the transition of HMH training protocols to clinical implementations.
Introduction
Human-human interaction
Humans often interact with each other while performing motor tasks, either to improve performance by working as a group or to learn from each other. Some motor tasks, such as exercising an injured joint, can be accomplished by a single person, but also allow two or more people (e.g., the therapist and the patient) to interact during performance of the task. Other motor tasks require multiple people to interact and cannot be performed by a single person (e.g., carrying a large table). Human-human (HH) interaction in tasks such as these can take the form of audiovisual communication and/or physical interaction.
In recent years, many studies have compared motor task performance under solo conditions and under different types of interaction among groups of two or more [1,2,3,4,5]. For tasks that can be performed with one or more people, we refer to task performance as the individual ability to accomplish a motor task during or without interaction. Similarly, for tasks that require multiple people, task performance is the ability to perform the task as a group.
There has been an increasing interest also for the effects of HH interaction on individual motor learning [2, 6,7,8] for motor tasks. In this article, we refer to individual motor learning as the solo task performance difference before and after a training period. This training phase can be performed individually or by interacting with someone else. In this review, we are interested in the learning of only individuals but not groups. Therefore, we do not analyze motor learning for tasks that require multiple people.
Studies that have demonstrated better outcomes of HH interaction than solo performance or training often attributed these positive effects to (1) increased motivation and interest [3, 9, 10]; (2) the ability to estimate partner’s motion, [11, 12]; and (3) the summing of physical effort, or the ability of partners to communicate and adopt specialized roles in performing a physical task [1, 13].
Even though important results have been obtained showing the potential benefits of human-human interaction, it is unclear how different kinds of interactions impact task performance and individual motor learning. A better understanding of how humans interact with each other in motor control tasks will have a significant impact on rehabilitation robotics, industrial collaborative robotics, and social robotics.[..]

[VIDEO] Finding Motor Points for Functional Electrical Stimulation in Hand Therapy
Posted by Kostas Pantremenos in Functional Electrical Stimulation (FES), Paretic Hand, REHABILITATION, Video on January 10, 2022
This Technique Peek video features Joanna Spivack, OTR/L, CHT demonstrating how to find motor points in order to provide a more functional electrical stimulation session. These techniques can be used in conjunction with exercises in order to enhance motor control, mobility and strength development.
[Abstract + References] Enhancing mirror therapy via scaling and shared control: a novel open-source virtual reality platform for stroke rehabilitation
Posted by Kostas Pantremenos in Mirror therapy, Virtual reality rehabilitation on October 19, 2021
Abstract
Mirror therapy is increasingly used in stroke rehabilitation to improve functional movements of the affected limb. However, the extent of mirroring in conventional mirror therapy is typically fixed (1:1) and cannot be tailored based on the patient’s impairment level. Further, the movements of the affected limb are not actively incorporated in the therapeutic process.
To address these issues, we developed an immersive VR system using HTC Vive and Leap Motion, which communicates with our free and open-source software environment programmed using SteamVR and the Unity 3D gaming engine. The mirror therapy VR environment was incorporated with two novel features: (1) scalable mirroring and (2) shared control. In the scalable mirroring, mirror movements were programmed to be scalable between 0 and 1, where 0 represents no movements, 0.5 represents 50% mirroring, and 1 represents 100% mirroring. In shared control, the contribution of the mirroring limb to the movements was programmed to be scalable between 0 to 1, where 0 represents 100% contribution from the mirroring limb (i.e., no mirroring), 0.5 represents 50% of movements from the mirrored limb and 50% of movements from the mirroring limb, and 1 represents full mirroring (i.e., no shared movements).
Validation experiments showed that these features worked appropriately. The proposed VR-based mirror therapy is the first fully developed system that is freely available to the rehabilitation science community. The scalable and shared control features can diversify mirror therapy and potentially augment the outcomes of rehabilitation, although this needs to be verified through future experiments.
References
- Abbink DA, Mulder M, Boer ER (2012) Haptic shared control: smoothly shifting control authority? Cogn Technol Work 14:19–28. https://doi.org/10.1007/s10111-011-0192-5Article Google Scholar
- Arya KN, Pandian S (2013) Effect of task-based mirror therapy on motor recovery of the upper extremity in chronic stroke patients: a pilot study. Top Stroke Rehabil 20:210–217. https://doi.org/10.1310/tscir2001-210Article Google Scholar
- Arya KN, Pandian S, Kumar D, Puri V (2015) Task-based mirror therapy augmenting motor recovery in poststroke hemiparesis: a randomized controlled trial. J Stroke Cerebrovasc Dis 24:1738–1748. https://doi.org/10.1016/j.jstrokecerebrovasdis.2015.03.026Article Google Scholar
- Arya KN, Pandian S, Kumar V (2019) Effect of activity-based mirror therapy on lower limb motor-recovery and gait in stroke: a randomised controlled trial. Neuropsychol Rehabil 29:1193–1210. https://doi.org/10.1080/09602011.2017.1377087Article Google Scholar
- Bai Z, Fong KNK, Zhang J, Hu Z (2020) Cortical mapping of mirror visual feedback training for unilateral upper extremity: a functional near-infrared spectroscopy study. Brain Behav. https://doi.org/10.1002/brb3.1489Article Google Scholar
- Broderick P, Horgan F, Blake C et al (2018) Mirror therapy for improving lower limb motor function and mobility after stroke: a systematic review and meta-analysis. Gait Posture 63:208–220. https://doi.org/10.1016/j.gaitpost.2018.05.017Article Google Scholar
- Crosbie JH, Lennon S, Basford JR, McDonough SM (2007) Virtual reality in stroke rehabilitation: still more virtual than real. Disabil Rehabil 29:1139–1146. https://doi.org/10.1080/09638280600960909Article Google Scholar
- Fritzsch C, Wang J, Dos Santos LF et al (2014) Different effects of the mirror illusion on motor and somatosensory processing. Restor Neurol Neurosci 32:269–280. https://doi.org/10.3233/RNN-130343Article Google Scholar
- Garry MI, Loftus A, Summers JJ (2005) Mirror, mirror on the wall: viewing a mirror reflection of unilateral hand movements facilitates ipsilateral M1 excitability. Exp Brain Res 163:118–122. https://doi.org/10.1007/s00221-005-2226-9Article Google Scholar
- González-Franco M, Pérez-Marcos D, Spanlang B, Slater M (2010) The contribution of real-time mirror reflections of motor actions on virtual body ownership in an immersive virtual environment. Proc IEEE Virtual Real. https://doi.org/10.1109/VR.2010.5444805Article Google Scholar
- Heinrich C, Cook M, Langlotz T, Regenbrecht H (2020) My hands? Importance of personalised virtual hands in a neurorehabilitation scenario. Virtual Real. https://doi.org/10.1007/s10055-020-00456-4Article Google Scholar
- Hoermann S, Ferreira Dos Santos L, Morkisch N et al (2017) Computerised mirror therapy with augmented reflection technology for early stroke rehabilitation: clinical feasibility and integration as an adjunct therapy. Disabil Rehabil 39(15):1503–1514. https://doi.org/10.1080/09638288.2017.1291765Article Google Scholar
- In T, Lee K, Song C (2016) Virtual reality reflection therapy improves balance and gait in patients with chronic stroke: randomized controlled trials. Med Sci Monit 22:4046–4053. https://doi.org/10.12659/msm.898157Article Google Scholar
- Kang YJ, Park HK, Kim HJ et al (2012) Upper extremity rehabilitation of stroke: facilitation of corticospinal excitability using virtual mirror paradigm. J Neuroeng Rehabil. https://doi.org/10.1186/1743-0003-9-71Article Google Scholar
- Kim K, Lee S, Kim D et al (2016) Effects of mirror therapy combined with motor tasks on upper extremity function and activities daily living of stroke patients. J Phys Ther Sci 28:483–487. https://doi.org/10.1589/jpts.28.483Article Google Scholar
- Kleim JA, Jones TA (2008) Principles of experience-dependent neural plasticity: Implications for rehabilitation after brain damage. J Speech Lang Hear Res 51
- Lee D, Lee M, Lee K, Song C (2014) Asymmetric training using virtual reality reflection equipment and the enhancement of upper limb function in stroke patients: a randomized controlled trial. J Stroke Cerebrovasc Dis 23:1319–1326. https://doi.org/10.1016/j.jstrokecerebrovasdis.2013.11.006Article Google Scholar
- Lu EC, Wang RH, Hebert D et al (2011) The development of an upper limb stroke rehabilitation robot: identification of clinical practices and design requirements through a survey of therapists. Disabil Rehabil Assist Technol 6:420–431. https://doi.org/10.3109/17483107.2010.544370Article Google Scholar
- Morales-Rodriguez ML, Pavard B (2007) Design of an emotional and social interaction paradigm for the animation of 3D characters: the case of a therapy for brain injured people (the mirror neuron paradigm). Virtual Real 11:175–184. https://doi.org/10.1007/s10055-006-0063-1Article Google Scholar
- Morkisch N, Thieme H, Dohle C (2019) How to perform mirror therapy after stroke? Evidence from a meta-analysis. Restor Neurol Neurosci 37:421–435. https://doi.org/10.3233/RNN-190935Article Google Scholar
- O’Sullivan N, de Bezenac C, Piovesan A et al (2018) I Am There … but Not Quite: an unfaithful mirror that reduces feelings of ownership and agency. Perception 47:197–215. https://doi.org/10.1177/0301006617743392Article Google Scholar
- Organization WH (2002) The world health report 2002: reducing risks, promoting healthy life
- Park Y, Chang M, Kim K-M, An D-H (2015) The effects of mirror therapy with tasks on upper extremity function and self-care in stroke patients. J Phys Ther Sci 27:1499–1501. https://doi.org/10.1589/jpts.27.1499Article Google Scholar
- Ramachandran VS, Rogers-Ramachandran D (2019) Mirror feedback assisted recovery from hemiparesis following stroke. in Reply to Morkisch et al.: How to perform mirror therapy after stroke? Evidence from a meta-analysis. Restor Neurol Neurosci 37:437–443Google Scholar
- Ramachandran VS, Rogers-Ramachandran D, Cobb S (1995) Touching the phantom limb. Nature 377:489–490. https://doi.org/10.1038/377489a0Article Google Scholar
- Ranganathan R (2017) Reorganization of finger coordination patterns through motor exploration in individuals after stroke. J Neuroeng Rehabil. https://doi.org/10.1186/s12984-017-0300-8Article Google Scholar
- Samuelkamaleshkumar S, Reethajanetsureka S, Pauljebaraj P et al (2014) Mirror therapy enhances motor performance in the paretic upper limb after stroke: a pilot randomized controlled trial. Arch Phys Med Rehabil 95:2000–2005. https://doi.org/10.1016/j.apmr.2014.06.020Article Google Scholar
- Sanchez-Vives MV, Slater M (2005) From presence to consciousness through virtual reality. Nat Rev Neurosci 6:332–339Article Google Scholar
- Subramanian SK, Levin MF (2011) Viewing medium affects arm motor performance in 3D virtual environments. J Neuroeng Rehabil. https://doi.org/10.1186/1743-0003-8-36Article Google Scholar
- Subramanian SK, Lourenço CB, Chilingaryan G et al (2013) Arm motor recovery using a virtual reality intervention in chronic stroke: randomized control trial. Neurorehabil Neural Repair 27:13–23. https://doi.org/10.1177/1545968312449695Article Google Scholar
- Subramanian SK, Cross MK, Hirschhauser CS (2020) Virtual reality interventions to enhance upper limb motor improvement after a stroke: commonly used types of platform and outcomes. Disabil Rehabil Assist Technol
- Thieme H, Morkisch N, Mehrholz J, et al (2018) Mirror therapy for improving motor function after stroke (Review) SUMMARY OF FINDINGS FOR THE MAIN COMPARIS1. Kawakami K, Miyasaka H, Nonoyama S, et al. Randomized controlled comparative study on effect of training to improve lower limb motor paralysis. Cochrane Database Syst Rev 1–182. doi:https://doi.org/10.1002/14651858.CD008449.pub3.www.cochranelibrary.com
- Virani SS, Alonso A, Benjamin EJ et al (2020) Heart disease and stroke statistics—2020 update: a report from the American Heart Association. Circulation 141:E139–E596Google Scholar
- Weber LM, Nilsen DM, Gillen G et al (2019) Immersive virtual reality mirror therapy for upper limb recovery after stroke: a pilot study. Am J Phys Med Rehabil 98:783–788. https://doi.org/10.1097/PHM.0000000000001190Article Google Scholar
- Zhu M-H, Zeng M, Shi M-F et al (2020) Visual feedback therapy for restoration of upper limb function of stroke patients. Int J Nurs Sci 7:170–178. https://doi.org/10.1016/j.ijnss.2020.04.004Article Google Scholar
[WEB PAGE] Using VR training to boost the sense of agency and improve motor control
Posted by Kostas Pantremenos in Virtual reality rehabilitation on January 28, 2021
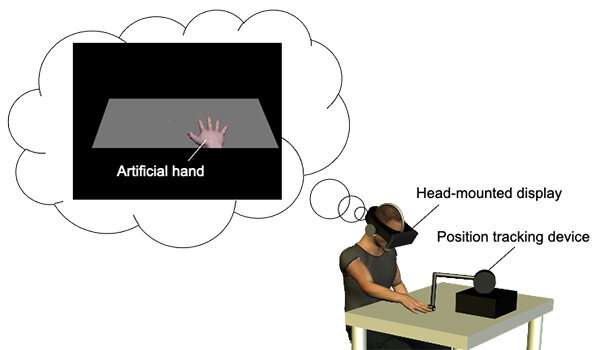
With Japan’s society rapidly aging, there has been a sharp increase in patients who experience motor dysfunctions. Rehabilitation is key to overcoming such ailments. A researcher from Tohoku University has developed a new virtual reality (VR) based method that can benefit rehabilitation and sports training by increasing bodily awareness and improving motor control.
His research was published in the journal Scientific Reports.
Not only can we see and touch our body, but we can sense it too. Our body is constantly firing off information to our brains that tell us where our limbs are in real-time. This process makes us aware of our body and gives us ownership over it. Meanwhile, our ability to control the movement and actions of our body parts voluntarily affords us agency over our body.
Ownership and agency are highly integrated and are related to our motor control. However, separating our sense of body ownership from our sense of agency has long evaded researchers, making it difficult to ascertain whether both ownership and agency truly affect motor control.
Professor Kazumichi Matsumiya from the Graduate School of Information Sciences at Tohoku University could isolate these two senses by using VR. Participants viewed a computer-generated hand, and Matsumiya independently measured their sense of ownership and agency over the hand.
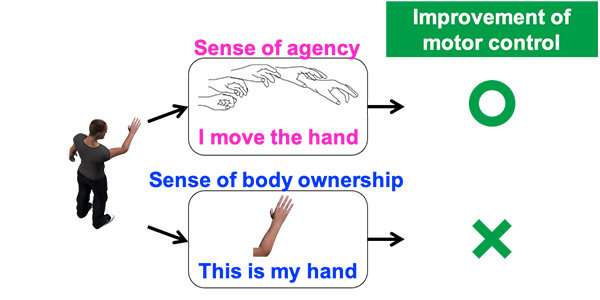
“I found that motor control is improved when participants experienced a sense of agency over the artificial body, regardless of their sense of body ownership,” said Matsumiya. “Our findings suggest that artificial manipulation of agency will enhance the effectiveness of rehabilitation and aid sports training techniques to improve overall motor control.”
[ARTICLE] Technology-aided assessment of functionally relevant sensorimotor impairments in arm and hand of post-stroke individuals – Full Text
Posted by Kostas Pantremenos in Paretic Hand on January 4, 2021
Abstract
Background
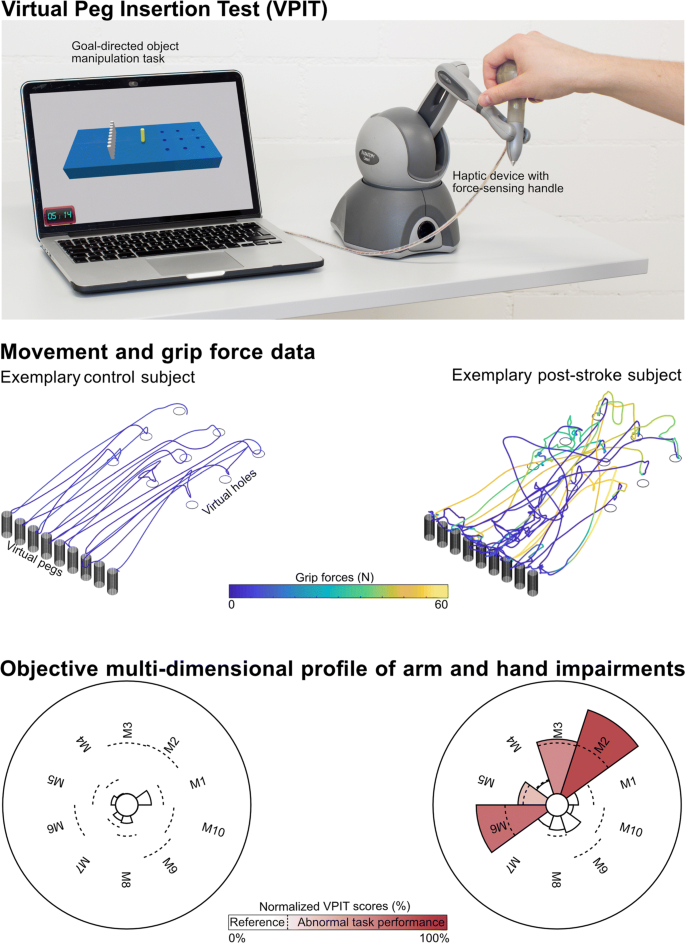
Assessing arm and hand sensorimotor impairments that are functionally relevant is essential to optimize the impact of neurorehabilitation interventions. Technology-aided assessments should provide a sensitive and objective characterization of upper limb impairments, but often provide arm weight support and neglect the importance of the hand, thereby questioning their functional relevance. The Virtual Peg Insertion Test (VPIT) addresses these limitations by quantifying arm and hand movements as well as grip forces during a goal-directed manipulation task requiring active lifting of the upper limb against gravity. The aim of this work was to evaluate the ability of the VPIT metrics to characterize arm and hand sensorimotor impairments that are relevant for performing functional tasks.
Methods
Arm and hand sensorimotor impairments were systematically characterized in 30 chronic stroke patients using conventional clinical scales and the VPIT. For the latter, ten previously established kinematic and kinetic core metrics were extracted. The validity and robustness of these metrics was investigated by analyzing their clinimetric properties (test-retest reliability, measurement error, learning effects, concurrent validity).
Results
Twenty-three of the participants, the ones with mild to moderate sensorimotor impairments and without strong cognitive deficits, were able to successfully complete the VPIT protocol (duration 16.6 min). The VPIT metrics detected impairments in arm and hand in 90.0% of the participants, and were sensitive to increased muscle tone and pathological joint coupling. Most importantly, significant moderate to high correlations between conventional scales of activity limitations and the VPIT metrics were found, thereby indicating their functional relevance when grasping and transporting objects, and when performing dexterous finger manipulations. Lastly, the robustness of three out of the ten VPIT core metrics in post-stroke individuals was confirmed.
Conclusions
This work provides evidence that technology-aided assessments requiring goal-directed manipulations without arm weight support can provide an objective, robust, and clinically feasible way to assess functionally relevant sensorimotor impairments in arm and hand in chronic post-stroke individuals with mild to moderate deficits. This allows for a better identification of impairments with high functional relevance and can contribute to optimizing the functional benefits of neurorehabilitation interventions.
Introduction
Stroke is a leading cause of acquired adult disability [1]. The incident commonly causes chronic sensorimotor deficits in arm and hand (impairments) [2, 3]. Impairments that are functionally relevant are especially critical for affected individuals, as these impairments reduce the spectrum of activities that an individual can perform (activity limitations) and determine the level of dependence on caregivers. Neurorehabilitation attempts to decrease the level of disability through inter-disciplinary interventions, including physical therapy [4, 5]. Achieving successful rehabilitation, with clear benefits for the independence of individuals typically requires the identification and therapy of functionally relevant impairments [6–8].
Conventional clinical scales are the current standard to evaluate upper limb sensorimotor impairments in research studies and the described impairments mostly show strong links to activity limitations (i.e., functional relevance) [9–13]. However, conventional assessments commonly rely on subjectively rated ordinal scales with ceiling effects that are not sensitive enough to detect fine changes in impairments and even introduce bias when attempting to model sensorimotor recovery [14–16]. Hence, providing a more objective assessment of functionally relevant sensorimotor impairments with sensitive scales should be of primary interest to neurorehabilitation researchers.
Digital health metrics extracted from technology-aided assessments can provide objective and traceable descriptions of upper limb behavior on sensitive, continuous scales without ceiling effects [17–19]. However, the majority of technology-aided assessments focus on characterizing impairments during planar arm movements while providing gravity support [20–23]. This neglects the importance of hand impairments and shadows the effects of certain deficits, such as weakness [19], which are both fundamental when performing daily activities. This questions the functional relevance of these assessments.
More recently, technology-aided approaches started emphasizing the importance of assessing impairments during tasks involving arm movements and hand manipulations without providing arm weight support [24–27]. Such tasks are expected to provide crucial information on fine upper limb impairments in individuals with mild to moderate disability levels and are promising to better identify functionally relevant impairments. However, existing approaches typically rely on time-consuming and complex measurement setups, which reduces their clinical applicability. Further, they mostly focus on kinematic metrics and do not quantify grip force control and its essential role in daily life activities [28, 29]. Also, the clinimetric properties of such digital health metrics are often insufficiently evaluated, thereby challenging their interpretability and acceptability as clinical endpoints [17, 30].
The Virtual Peg Insertion Test (VPIT) addresses many of the limitations of existing technology-aided assessments by recording movement and grip force patterns during a virtual goal-directed manipulation task requiring coordinated arm and hand movements [31, 32]. Previous research indicated the feasibility of the approach in neurologic individuals with mild to moderate sensorimotor impairments [32–35]. In addition, ten digital health metrics capturing sensorimotor impairments have been established for the VPIT and allowed for an accurate discrimination between neurologically intact and affected individuals [32]. However, whether the VPIT metrics provide a multi-dimensional evaluation of impairments in arm and hand that are functionally relevant has not been evaluated yet. Further, the clinimetric properties (test-retest reliability, measurement error, learning effects, concurrent validity) of the VPIT metrics have mainly been evaluated in unaffected subjects, thereby leaving their applicability and robustness in post-stroke individuals unexplored.
The objective of this work was to evaluate the ability of the digital health metrics from the VPIT to characterize arm and hand sensorimotor impairments that are relevant for performing functional tasks, by evaluating their clinimetric properties in 30 chronic post-stroke subjects.
Methods
Virtual Peg Insertion Test (VPIT)
The VPIT (Fig. 1, video at https://youtu.be/TyJyd5uVN68) as an upper limb sensorimotor assessment has been described in detail in previous work [31–33]. In short, it consists of a commercial haptic end-effector device (PhantomOmni or Geomagic Touch, 3D Systems, USA), a rapid-prototyped grasping force sensing handle, and a virtual reality environment on a personal computer (total material costs approximately 4000 USD). The virtual reality environment displays a virtual pegboard task that requires the insertion of nine virtual pegs into nine holes. The pegboard has dimensions similar to the Nine Hole Peg Test (26.8 ×12.8 ×6.2 cm) [36]. More specifically, a virtual cursor can be controlled through the coordination of end-effector movements and applied grasping force. To pick up a peg, the cursor first needs to be spatially aligned with the peg. Subsequently, a grasping force of at least 2N has to be maintained to transport the peg towards a hole. The peg can be released in a hole upon a reduction of the grasping force below 2N. Based on this task design, the VPIT engages various aspects of sensorimotor control and assesses goal-directed arm movements, while actively lifting the arm against gravity, in combination with spherical grip force control. Hence, the VPIT should be seen as a hybrid solution, combining elements of the Nine Hole Peg Test (NHPT) and the Box and Block Test (BBT). This is expected to provide a multi-dimensional picture of different sensorimotor impairments in a functional context.
[Abstract + References] Movement Quality: A Novel Biomarker Based on Principles of Neuroscience
Posted by Kostas Pantremenos in Uncategorized on November 30, 2020
Abstract
A major problem in neurorehabilitation is the lack of objective outcomes to measure movement quality. Movement quality features, such as coordination and stability, are essential for everyday motor actions. These features allow reacting to continuously changing environment or to resist external perturbations. Neurological disorders affect movement quality, leading to functionally impaired movements. Recent findings suggest that the central nervous system organizes motor elements (eg, muscles, joints, fingers) into task-specific ensembles to stabilize motor tasks performance. A method to quantify this feature has been previously developed based on the uncontrolled manifold (UCM) hypothesis. UCM quantifies movement quality in a spatial-temporal domain using intertrial analysis of covariation between motor elements. In this point-of-view article, we first describe major obstacles (eg, the need for group analysis) that interfere with UCM application in clinical settings. Then, we propose a process of quantifying movement quality for a single individual with a novel use of bootstrapping simulations and UCM analysis. Finally, we reanalyze previously published data from individuals with neurological disorders performing a wide range of motor tasks, that is, multi-digit pressing and postural balance tasks. Our method allows one to assess motor quality impairments in a single individual and to detect clinically important motor behavior changes. Our solution may be incorporated into a clinical setting to assess sensorimotor impairments, evaluate the effects of specific neurological treatments, or track movement quality recovery over time. We also recommended the proposed solution to be used jointly with a typical statistical analysis of UCM parameters in cohort studies.
References
| 1. | Stinear, CM, Lang, CE, Zeiler, S, Byblow, WD. Advances and challenges in stroke rehabilitation. Lancet Neurol. 2020;19:348-360. doi:10.1016/S1474-4422(19)30415-6 Google Scholar | Crossref | Medline |
| 2. | Clarke, CE, Patel, S, Ives, N, et al; PD REHAB Collaborative Group . Physiotherapy and occupational therapy vs no therapy in mild to moderate Parkinson disease: a randomized clinical trial. JAMA Neurol. 2016;73:291-299. doi:10.1001/jamaneurol.2015.4452 Google Scholar | Crossref | Medline |
| 3. | French, B, Thomas, LH, Coupe, J, et al. Repetitive task training for improving functional ability after stroke. Cochrane Database Syst Rev. 2016;11:CD006073. doi:10.1002/14651858.CD006073.pub3 Google Scholar | Crossref | Medline |
| 4. | Dobkin, B, Apple, D, Barbeau, H, et al; Spinal Cord Injury Locomotor Trial Group . Weight-supported treadmill vs over-ground training for walking after acute incomplete SCI. Neurology. 2006;66:484-493. doi:10.1212/01.wnl.0000202600.72018.39 Google Scholar | Crossref | Medline | ISI |
| 5. | Piscitelli, D. Motor rehabilitation should be based on knowledge of motor control. Arch Physiother. 2016;6:5. doi:10.1186/s40945-016-0019-z Google Scholar | Crossref | Medline |
| 6. | Stinear, CM. Stroke rehabilitation research needs to be different to make a difference. F1000Res. 2016;5:F1000 Faculty Rev 1467. doi:10.12688/f1000research.8722.1 Google Scholar | Crossref | Medline |
| 7. | Winstein, CJ, Wolf, SL, Dromerick, AW, et al. Effect of a task-oriented rehabilitation program on upper extremity recovery following motor stroke: the ICARE randomized clinical trial. JAMA. 2016;315:571-581. doi:10.1001/jama.2016.0276 Google Scholar | Crossref | Medline | ISI |
| 8. | Piscitelli, D. Neurorehabilitation: bridging neurophysiology and clinical practice. Neurol Sci. 2019;40:2209-2211. doi:10.1007/s10072-019-03969-2 Google Scholar | Crossref | Medline |
| 9. | Latash, ML, Huang, X. Neural control of movement stability: Lessons from studies of neurological patients. Neuroscience. 2015;301:39-48. doi:10.1016/j.neuroscience.2015.05.075 Google Scholar | Crossref | Medline |
| 10. | Kleim, JA, Jones, TA. Principles of experience-dependent neural plasticity: implications for rehabilitation after brain damage. J Speech Lang Hear Res. 2008;51(1):S225-S239. doi:10.1044/1092-4388(2008/018) Google Scholar | Crossref | Medline | ISI |
| 11. | Winstein, CJ, Wing, AM, Whitall, J. Motor control and learning principles for rehabilitation of upper limb movements after brain injury. In: Grafman, J, Robertson, LH, eds. Handbook of Neuropsychology. 2nd ed. Vol 9. Elsevier; 2003:79-138. Google Scholar |
| 12. | Krakauer, JW, Carmichael, ST, Corbett, D, Wittenberg, GF. Getting neurorehabilitation right: what can be learned from animal models? Neurorehabil Neural Repair. 2012;26:923-931. doi:10.1177/1545968312440745 Google Scholar | SAGE Journals | ISI |
| 13. | Schmidt, RA, Lee, TD, Winstein, CJ, Wulf, G, Zelaznik, HN. Motor Control and Learning: A Behavioral Emphasis. 6th ed. Human Kinetics; 2019. Google Scholar |
| 14. | Tomita, Y, Rodrigues, MRM, Levin, MF. Upper limb coordination in individuals with stroke: poorly defined and poorly quantified. Neurorehabil Neural Repair. 2017;31:885-897. doi:10.1177/1545968317739998 Google Scholar | SAGE Journals | ISI |
| 15. | Levin, MF, Kleim, JA, Wolf, SL. What do motor “recovery” and “compensation” mean in patients following stroke? Neurorehabil Neural Repair. 2009;23:313-319. doi:10.1177/1545968308328727 Google Scholar | SAGE Journals | ISI |
| 16. | Demers, M, Levin, MF. Do activity level outcome measures commonly used in neurological practice assess upper-limb movement quality? Neurorehabil Neural Repair. 2017;31:623-637. doi:10.1177/1545968317714576 Google Scholar | SAGE Journals | ISI |
| 17. | Tomita, Y, Mullick, AA, Levin, MF. Reduced kinematic redundancy and motor equivalence during whole-body reaching in individuals with chronic stroke. Neurorehabil Neural Repair. 2018;32:175-186. doi:10.1177/1545968318760725 Google Scholar | SAGE Journals | ISI |
| 18. | Kwakkel, G, van Wegen, EEH, Burridge, JH, et al; ADVISORY Group . Standardized measurement of quality of upper limb movement after stroke: consensus-based core recommendations from the second stroke recovery and rehabilitation roundtable. Neurorehabil Neural Repair. 2019;33:951-958. doi:10.1177/1545968319886477 Google Scholar | SAGE Journals | ISI |
| 19. | Asakawa, T, Fang, H, Sugiyama, K, et al. Human behavioral assessments in current research of Parkinson’s disease. Neurosci Biobehav Rev. 2016;68:741-772. doi:10.1016/j.neubiorev.2016.06.036 Google Scholar | Crossref | Medline |
| 20. | Latash, ML, Scholz, JP, Schoner, G. Toward a new theory of motor synergies. Motor Control. 2007;11:276-308. doi:10.1123/mcj.11.3.276 Google Scholar | Crossref | Medline | ISI |
| 21. | Latash, ML. Towards physics of neural processes and behavior. Neurosci Biobehav Rev. 2016;69:136-146. doi:10.1016/j.neubiorev.2016.08.005 Google Scholar | Crossref | Medline |
| 22. | d’Avella, A, Saltiel, P, Bizzi, E. Combinations of muscle synergies in the construction of a natural motor behavior. Nat Neurosci. 2003;6:300-308. doi:10.1038/nn1010 Google Scholar | Crossref | Medline |
| 23. | Sawner, KA, LaVigne, JM, Brunnstrom, S. Brunnstrom’s Movement Therapy in Hemiplegia: A Neurophysiological Approach. 2nd ed. Lippincott; 1992. Google Scholar |
| 24. | Dewald, JP, Pope, PS, Given, JD, Buchanan, TS, Rymer, WZ. Abnormal muscle coactivation patterns during isometric torque generation at the elbow and shoulder in hemiparetic subjects. Brain. 1995;118 (pt 2):495-510. doi:10.1093/brain/118.2.495 Google Scholar | Crossref | Medline |
| 25. | Latash, ML, Zatsiorsky, VM. Biomechanics and Motor Control: Defining Central Concepts. Elsevier/Academic Press; 2016. Google Scholar |
| 26. | Levin, MF, Nichols, TR, Jaric, S. Progress in Motor Control VI conference. Motor Control. 2007;11(suppl). Google Scholar |
| 27. | Scholz, JP, Schoner, G. The uncontrolled manifold concept: identifying control variables for a functional task. Exp Brain Res. 1999;126:289-306. doi:10.1007/s002210050738 Google Scholar | Crossref | Medline | ISI |
| 28. | Olafsdottir, H, Yoshida, N, Zatsiorsky, VM, Latash, ML. Anticipatory covariation of finger forces during self-paced and reaction time force production. Neurosci Lett. 2005;381:92-96. doi:10.1016/j.neulet.2005.02.003 Google Scholar | Crossref | Medline | ISI |
| 29. | Klous, M, Mikulic, P, Latash, ML. Two aspects of feedforward postural control: anticipatory postural adjustments and anticipatory synergy adjustments. J Neurophysiol. 2011;105:2275-2288. doi:10.1152/jn.00665.2010 Google Scholar | Crossref | Medline |
| 30. | Piscitelli, D, Falaki, A, Solnik, S, Latash, ML. Anticipatory postural adjustments and anticipatory synergy adjustments: preparing to a postural perturbation with predictable and unpredictable direction. Exp Brain Res. 2017;235:713-730. doi:10.1007/s00221-016-4835-x Google Scholar | Crossref | Medline |
| 31. | Vaz, DV, Pinto, VA, Junior, RRS, Mattos, DJS, Mitra, S. Coordination in adults with neurological impairment—a systematic review of uncontrolled manifold studies. Gait Posture. 2019;69:66-78. doi:10.1016/j.gaitpost.2019.01.003 Google Scholar | Crossref | Medline |
| 32. | Lewis, MM, Lee, EY, Jo, HJ, et al. Synergy as a new and sensitive marker of basal ganglia dysfunction: a study of asymptomatic welders. Neurotoxicology. 2016;56:76-85. doi:10.1016/j.neuro.2016.06.016 Google Scholar | Crossref | Medline |
| 33. | Park, J, Lewis, MM, Huang, X, Latash, ML. Effects of olivo-ponto-cerebellar atrophy (OPCA) on finger interaction and coordination. Clin Neurophysiol. 2013;124:991-998. doi:10.1016/j.clinph.2012.10.021 Google Scholar | Crossref | Medline | ISI |
| 34. | Park, J, Wu, YH, Lewis, MM, Huang, X, Latash, ML. Changes in multifinger interaction and coordination in Parkinson’s disease. J Neurophysiol. 2012;108:915-924. doi:10.1152/jn.00043.2012 Google Scholar | Crossref | Medline | ISI |
| 35. | Falaki, A, Huang, X, Lewis, MM, Latash, ML. Dopaminergic modulation of multi-muscle synergies in postural tasks performed by patients with Parkinson’s disease. J Electromyogr Kinesiol. 2017;33:20-26. doi:10.1016/j.jelekin.2017.01.002 Google Scholar | Crossref | Medline |
| 36. | Cuadra, C, Falaki, A, Sainburg, R, Sarlegna, FR, Latash, ML. Case Studies in Neuroscience: The central and somatosensory contributions to finger interdependence and coordination: lessons from a study of a “deafferented person. ” J Neurophysiol. 2019;121:2083-2087. doi:10.1152/jn.00153.2019 Google Scholar | Crossref | Medline |
| 37. | Reisman, DS, Scholz, JP. Workspace location influences joint coordination during reaching in post-stroke hemiparesis. Exp Brain Res. 2006;170:265-276. doi:10.1007/s00221-005-0209-5 Google Scholar | Crossref | Medline | ISI |
| 38. | Falaki, A, Jo, HJ, Lewis, MM, et al. Systemic effects of deep brain stimulation on synergic control in Parkinson’s disease. Clin Neurophysiol. 2018;129:1320-1332. doi:10.1016/j.clinph.2018.02.126 Google Scholar | Crossref | Medline |
| 39. | Mattos, DJ, Latash, ML, Park, E, Kuhl, J, Scholz, JP. Unpredictable elbow joint perturbation during reaching results in multijoint motor equivalence. J Neurophysiol. 2011;106:1424-1436. doi:10.1152/jn.00163.2011 Google Scholar | Crossref | Medline |
| 40. | Mattos, D, Kuhl, J, Scholz, JP, Latash, ML. Motor equivalence (ME) during reaching: is ME observable at the muscle level? Motor Control. 2013;17:145-175. doi:10.1123/mcj.17.2.145 Google Scholar | Crossref | Medline |
| 41. | Solnik, S, Pazin, N, Coelho, CJ, et al. End-state comfort and joint configuration variance during reaching. Exp Brain Res. 2013;225:431-442. doi:10.1007/s00221-012-3383-2 Google Scholar | Crossref | Medline |
| 42. | Solnik, S, Reschechtko, S, Wu, YH, Zatsiorsky, VM, Latash, ML. Interpersonal synergies: static prehension tasks performed by two actors. Exp Brain Res. 2016;234:2267-2282. doi:10.1007/s00221-016-4632-6 Google Scholar | Crossref | Medline |
| 43. | Furmanek, MP, Solnik, S, Piscitelli, D, Rasouli, O, Falaki, A, Latash, ML. Synergies and motor equivalence in voluntary sway tasks: the effects of visual and mechanical constraints. J Mot Behav. 2018;50:492-509. doi:10.1080/00222895.2017.1367642 Google Scholar | Crossref | Medline |
| 44. | Papi, E, Rowe, PJ, Pomeroy, VM. Analysis of gait within the uncontrolled manifold hypothesis: stabilisation of the centre of mass during gait. J Biomech. 2015;48:324-331. doi:10.1016/j.jbiomech.2014.11.024 Google Scholar | Crossref | Medline |
| 45. | Rosenblatt, NJ, Hurt, CP. Recommendation for the minimum number of steps to analyze when performing the uncontrolled manifold analysis on walking data. J Biomech. 2019;85:218-223. doi:10.1016/j.jbiomech.2019.01.018 Google Scholar | Crossref | Medline |
| 46. | Gajdosik, RL, Bohannon, RW. Clinical measurement of range of motion. Review of goniometry emphasizing reliability and validity. Phys Ther. 1987;67:1867-1872. doi:10.1093/ptj/67.12.1867 Google Scholar | Crossref | Medline | ISI |
| 47. | Efron, B, Tibshirani, R. An Introduction to the Bootstrap. Chapman & Hall; 1993. Google Scholar | Crossref |
| 48. | Efron, B. Better bootstrap confidence intervals. J Am Stat Assoc. 1987;82:171-185. doi:10.1080/01621459.1987.10478410 Google Scholar | Crossref | ISI |
| 49. | Poitras, I, Dupuis, F, Bielmann, M, et al. Validity and reliability of wearable sensors for joint angle estimation: a systematic review. Sensors (Basel). 2019;19:1555. doi:10.3390/s19071555 Google Scholar | Crossref |
| 50. | Heywood, S, Pua, YH, McClelland, J, et al. Low-cost electromyography—validation against a commercial system using both manual and automated activation timing thresholds. J Electromyogr Kinesiol. 2018;42:74-80. doi:10.1016/j.jelekin.2018.05.010 Google Scholar | Crossref | Medline |
| 51. | Supuk, TG, Skelin, AK, Cic, M. Design, development and testing of a low-cost sEMG system and its use in recording muscle activity in human gait. Sensors (Basel). 2014;14:8235-8258. doi:10.3390/s140508235 Google Scholar | Crossref | Medline |
| 52. | Gorter, R, Fox, JP, Apeldoorn, A, Twisk, J. Measurement model choice influenced randomized controlled trial results. J Clin Epidemiol. 2016;79:140-149. doi:10.1016/j.jclinepi.2016.06.011 Google Scholar | Crossref | Medline |
[ARTICLE] The Basal Ganglia: More than just a switching device – Full Text
Posted by Kostas Pantremenos in Educational on November 25, 2020
Summary
The basal ganglia consist of a variety of subcortical nuclei engaged in motor control and executive functions, such as motor learning, behavioral control, and emotion. The striatum, a major basal ganglia component, is particularly useful for cognitive planning of purposive motor acts owing to its structural features and the neuronal circuitry established with the cerebral cortex. Recent data indicate emergent functions played by the striatum. Indeed, cortico‐striatal circuits carrying motor information are paralleled by circuits originating from associative and limbic territories, which are functionally integrated in the striatum. Functional integration between brain areas is achieved through patterns of coherent activity. Coherence belonging to cortico‐basal ganglia circuits is also present in Parkinson’s disease patients. Excessive synchronization occurring in this pathology is reduced by dopaminergic therapies. The mechanisms through which the dopaminergic effects may be addressed are the object of several ongoing investigations. Overall, the bulk of data reported in recent years has provided new vistas concerning basal ganglia role in the organization and control of movement and behavior, both in physiological and pathological conditions. In this review, basal ganglia functions involved in the organization of main movement categories and behaviors are critically discussed. Comparatively, the multiplicity of Parkinson’s disease symptomatology is also revised.
1 INTRODUCTION
The components of the basal ganglia (BG) system, including the striatum, are bilateral structures that, like the thalamus, serve behavior and movement control through the regulation of cortical output. The BG operate in close relation with the cerebral cortex being part of an extensive loop, the BG‐thalamic‐cortical system, and their association results in several processing circuits between different cortical areas.1, 2 Parallel cortical‐BG‐thalamocortical information flows through two pathways having opposite effects, the direct and indirect pathways,3 whose functioning is crucial for the proper execution of movement. Choosing the contextually appropriate response in the presence of competing alternatives is a critical aspect of motor control.4, 5 Direct and indirect pathways provide the neural mechanism to rapidly switch from a planned to an alternative response.6 The main BG input nucleus, through which the direct and indirect pathways manage cortical information, is the striatum that receives topographical excitatory projections from almost the entire cortical areas. Recent data indicate emergent functions played by the striatum. Indeed, cortico‐striatal circuits carrying motor information are paralleled by circuits originating from associative and limbic territories that are functionally integrated in the striatum. The striatal ability to switch from competing sensory‐motor processes, in response to specific context and situations, is based on its capability in processing, routing, and reverting cortical information.7, 8 Owing to its structural features and the neuronal circuitry established with the cerebral cortex, the striatum is particularly useful for cognitive planning of purposive motor acts. The peculiar striatal regional differentiation, as visible from histochemical evidence, and the circuitry features enable the BG system to operate a functional integration between brain areas.
Overall, the bulk of data reported in recent years has provided new vistas concerning BG role in the organization and control of movement and behavior, both in physiological and pathological conditions. In this review, BG functions involved in the organization of main movement categories and behaviors are critically discussed. Comparatively, the multiplicity of Parkinson’s disease (PD) symptomatology is also revised.[…]
[ARTICLE] Multiple processes independently predict motor learning – Full Text
Posted by Kostas Pantremenos in Neuroplasticity, Paretic Hand on November 18, 2020
Abstract
Background
Our ability to acquire, refine and adapt skilled limb movements is a hallmark of human motor learning that allows us to successfully perform many daily activities. The capacity to acquire, refine and adapt other features of motor performance, such as visual search, eye-hand coordination and visuomotor decisions, may also contribute to motor learning. However, the extent to which refinements of multiple behavioral features and their underlying neural processes independently contribute to motor learning remains unknown. In the current study, we used an ethological approach to test the hypothesis that practice-related refinements of multiple behavioral features would be independently predictive of motor learning.
Methods
Eighteen healthy, young adults used an upper-limb robot with eye-tracking to practice six trials of a continuous, visuomotor task once a week for six consecutive weeks. Participants used virtual paddles to hit away 200 “Targets” and avoid hitting 100 “Distractors” that continuously moved towards them from the back of the workspace. Motor learning was inferred from trial-by-trial acquisition and week-by-week retention of improvements on two measures of task performance related to motor execution and motor inhibition. Adaptations involving underlying neural processes were inferred from trial-by-trial acquisition and week-by-week retention of refinements on measures of skilled limb movement, visual search, eye-hand coordination and visuomotor decisions. We tested our hypothesis by quantifying the extent to which refinements on measures of multiple behavioral features (predictors) were independently predictive of improvements on our two measures of task performance (outcomes) after removing all shared variance between predictors.
Results
We found that refinements on measures of skilled limb movement, visual search and eye-hand coordination were independently predictive of improvements on our measure of task performance related to motor execution. In contrast, only refinements of eye-hand coordination were independently predictive of improvements on our measure of task performance related to motor inhibition.
Conclusion
Our results provide indirect evidence that refinements involving multiple, neural processes may independently contribute to motor learning, and distinct neural processes may underlie improvements in task performance related to motor execution and motor inhibition. This also suggests that refinements involving multiple, neural processes may contribute to motor recovery after stroke, and rehabilitation interventions should be designed to produce refinements of all behavioral features that may contribute to motor recovery.[…]
[ARTICLE] The effects of anxiety and dual-task on upper limb motor control of chronic stroke survivors – Full Text
Posted by Kostas Pantremenos in Paretic Hand on October 22, 2020
Abstract
This study was designed to investigate the effects of anxiety and dual-task on reach and grasp motor control in chronic stroke survivors compared with age- and sex-matched healthy subjects (HC). Reach and grasp kinematic data of 68 participants (high-anxiety stroke (HA-stroke), n = 17; low-anxiety stroke (LA-stroke), n = 17; low-anxiety HC, n = 17; and high-anxiety HC, n = 17) were recorded under single- and dual-task conditions. Inefficient reach and grasp of stroke participants, especially HA-stroke were found compared with the control groups under single- and dual-task conditions as evidenced by longer movement time (MT), lower and earlier peak velocity (PV) as well as delayed and smaller hand opening. The effects of dual-task on reach and grasp kinematic measures were similar between HCs and stroke participants (i.e., increased MT, decreased PV that occurred earlier, and delayed and decreased hand opening), with greater effect in stroke groups than HCs, and in HA-stroke group than LA-stroke group. The results indicate that performing a well-learned upper limb movement with concurrent cognitive task leads to decreased efficiency of motor control in chronic stroke survivors compared with HCs. HA-stroke participants were more adversely affected by challenging dual-task conditions, underlying importance of assessing anxiety and designing effective interventions for it in chronic stroke survivors.
Introduction
Approximately 60% of stroke survivors suffer from permanent upper limb dysfunctions despite receiving rehabilitation1. Stroke-induced motor impairments (e.g., muscle weakness, spasticity, and impaired coordination), sensory deficits (proprioceptive and/or tactile sensory loss) and perceptual-cognitive dysfunctions (e.g., attentional problems and visuospatial impairments), as well as secondary physiological adaptations (e.g., contractures, and muscle atrophy) can directly affect skilled/well-learned upper limb movements such as reach and grasp2. Slowed and segmented movement, dysmetria, inadequate aperture and impairments of hand preshaping have been reported as common problems, involved in clumsy function or disuse of the upper limb following stroke2,3,4,5. Reach and grasp, a fundamental part of object manipulation, requires the integration of sensory, motor and cognitive information6,7 and frequently performed with a concurrent cognitive task (e.g., reach and grasp of a cup of coffee while talking on the phone or reach and grasp goods from store shelves while recalling shopping list8.
Typically, a dual-task paradigm is used to investigate whether and to what extent control of a motor action requires attentional resources. Based on limited processing capacity theory, if a motor and cognitive task compete for shared attentional resources, performing the two task simultaneously may result in disruption of performance in one or both task, known as dual-task interference (for example, enhanced error and slower performance compared with the single-task condition)9. Individuals are frequently challenged by dual-task conditions in daily life, hence, flexible adaptation to the changing motor and cognitive requirements of daily functions, as well as environment is necessary for successfully and independently performing activities of daily livings (ADLs)10.
Stroke survivors may experience greater dual-task interference compared to healthy subjects because of impaired cognitive and motor function11. Although the effects of dual-task have been widely studied on balance and gait in stroke survivors11,12, few studies have been conducted on the effects of dual-task on upper limb function of these patients. In this regard, Shin et al. reported a significant dual-task effect on upper limb movement smoothness and reach error in chronic stroke survivors using a robotic-assisted planar reaching. However, they did not compare stroke survivors with healthy participants10. Bank et al. used a virtual goal-directed upper limb movement (i.e., controlling the movement of the virtual mouse to collect virtual targets), which performed with or without auditory stroop task in order to compare the cognitive-motor interference in patients with neurological disorders (stroke and Parkinson’s disease) with sex-and age-matched healthy controls. They did not find greater cognitive-motor interference in stroke participants than the healthy controls. They explained this finding might be related to their measure that was not precise. They suggested using a more precise measure for assessing upper limb motor control such as a motion analysis system13. Houwink et al. used a motion analysis system to investigate the effect of an auditory stroop task on upper limb motor control in chronic stroke survivors while drawing a circle. They found dual-task interference only in the affected upper limb of patients who had moderate upper limb paresis. However, they used an experimentally designed upper limb task, not a natural everyday task such as reach and grasp, which based on their stated limitation is susceptible to learning that is different among stroke survivors and healthy subjects14. It remains to be determined, however, whether dual-task would affect motor control of a well-learned/skillful upper limb movement such as reach and grasp in chronic stroke survivors.
Anxiety is the second most common psychological disorders among stroke survivors15, affecting up to 24% of patients15,16. It has been suggested that anxiety symptoms persist for up to 10 years after stroke17 and are associated with low functional outcomes17, increased dependency in ADLs18, and decreased quality of life17. Anxiety increases distraction by task-irrelevant stimuli (i.e., impaired attentional control), leading to decreased processing efficiency needed for motor planning and execution of a well-learned/skillful movement19. Kotani et al. recently showed that anxiety disrupted the hand’s fine motor control of expert pianists through incoordination of multi-joints movements20. Despite the high prevalence of anxiety in stroke survivors and its potential to affect motor control, to the best of our knowledge, no attention has been paid to the effects of anxiety on upper limb motor control of these patients. Understanding the effects of anxiety on upper limb motor control is therefore needed to develop and target interventions to address this common psychological disorder, improve upper limb motor control, and enhance the independence of stroke survivors in ADLs. Therefore, the aim of this study was to investigate whether dual-task interference would be observed in upper limb motor control of stroke survivors when performing a well-learned everyday motor task compared with age-and sex-matched healthy subjects. The study also aimed to determine the effect of anxiety on upper limb motor control of these patients.[…]
[Abstract] Chronic post-stroke deficits in gross and fine motor control of the ipsilesional upper extremity
Posted by Kostas Pantremenos in Paretic Hand on September 6, 2020
Abstract
Individuals with stroke often experience contralesional and ipsilesional arm motor deficits.
Objectives
Compare fine and gross motor hand dexterity of the ipsilesional hand post-stroke with controls, normative values, and the contralesional hand.
Design
Data were collected from right-handed individuals with chronic stroke (n = 20), age/sex matched controls (n = 10), and normative values (n = 20) performing the Nine Hole Peg Test and the Box and Blocks Test.
Results
Individuals with stroke demonstrated poorer performance with the ipsilesional arm relative to both the control group (mean difference [95% CI]: Nine Hole Peg Test (s): 3.4 [-0.5, 7.3]; Box and Blocks Test (# blocks): -12.3 [-20.3, -4.2]) and normative values (mean difference [95% CI]: Nine Hole Peg Test (s): 6.5 [4.0, 9.1]; Box and Blocks Test (# blocks): -15.3 [-20.1, -10.5]). Ipsilesional arm performance was significantly better than performance with the contralesional arm (mean difference [95% CI]: Nine Hole Peg Test (s): -9.4 [-20.2, 1.4]; Box and Blocks Test (# blocks): 33.2 [20.9, 45.5]).
Conclusion
These findings identify residual deficits in fine and gross dexterity of the ipsilesional hand in commonly used outcome measures of hand manipulation among individuals with chronic stroke. Possible underlying mechanisms and clinical relevance are discussed.
