Posts Tagged tDCS
[Preprint] Feasibility of Simultaneous Transcranial Direct Current Stimulation During Gait Training in Chronic Stroke Patients: A Randomized Double-blind Pilot Clinical Trial
Posted by Kostas Pantremenos in Gait Rehabilitation - Foot Drop, tDCS/rTMS on April 2, 2024
Abstract
Background
Transcranial direct current stimulation (tDCS) is a therapeutic tool for improving post-stroke gait
disturbances, with ongoing research focusing on specific protocols for its application. We evaluated the
feasibility of a rehabilitation protocol that combines tDCS with conventional gait training.
Methods
This was a randomized, double-blind, single-center pilot clinical trial. Patients with unilateral hemiplegia
due to ischemic stroke were randomly assigned to either the tDCS with gait training group or the sham
stimulation group. The anodal tDCS electrode was placed on the tibialis anterior area of the precentral
gyrus while gait training proceeded. Interventions were administered 3 times weekly for 4 weeks.
Outcome assessments, using the 10-meter walk test, Timed Up and Go test, Berg Balance Scale,
Functional Ambulatory Scale, Modified Barthel Index, and EQ-5D-3L, were conducted before and after the
intervention and again at the 8-week mark following its completion. Repeated-measures ANOVA was used for comparisons between and within groups.
Results
Twenty-six patients were assessed for eligibility, and 20 were enrolled and randomized. No significant
differences were observed between the tDCS with gait training group and the sham stimulation group in
gait speed after the intervention. However, the tDCS with gait training group showed significant
improvement in balance performance in both within-group and between-group comparisons. In the
subgroup analysis of patients with elicited motor-evoked potentials, comfortable pace gait speed
improved in the tDCS with gait training group. No serious adverse events occurred throughout the study.
Conclusions
Simultaneous tDCS during gait training is a feasible rehabilitation protocol for chronic stroke patients
with gait disturbances.
Introduction
Impairment of independent gait is one of the most disabling consequences after a stroke [1]. Gait
abnormality in stroke patients arises from a complex interplay of factors, including lower limb motor
weakness and decreased balance. Some individuals may not be entirely incapable of walking, but they
may still require gait aids or assistance from caregivers. Gait disturbances pose a greater risk of further
injury due to falls. It is well known that fall-associated fractures result in significant socioeconomic costs
[2]. Additionally, gait disturbances lead to limitations in social activity, thereby reducing the quality of life
of stroke patients. Therefore, improving gait performance in stroke patients has long been a desire shared
by patients and physicians.
Transcranial direct current stimulation (tDCS) is a non-invasive brain stimulation technique that aims to
modulate the human brain by delivering low-intensity electrical current through the scalp. The mechanism of tDCS is explained by 2 principles: (1) the enhancement of cortical activity through a polarity shift in the resting membrane potential and (2) the upregulation of neural plasticity through long-term potentiation [3]. Applying anodal tDCS to patients with subacute stroke has been associated with beneficial effects on motor function [4]. However, inconsistent results have emerged across studies, with some failing to observe significant improvements in patients who underwent tDCS compared to sham stimulation [5].
Additionally, there has been substantial variability in factors, such as stimulation area, intensity, duration,
and the number of sessions among different tDCS protocols, highlighting the need for further research to
establish an optimal tDCS protocol for maximizing the effect of conventional gait training methods.
Studies focusing on stimulation timing suggest that combining tDCS with gait training simultaneously
shows more promising results in improving gait performance compared to protocols that administer gait
training and tDCS separately [6].
This study aimed to evaluate the feasibility of a rehabilitation protocol that combines simultaneous tDCS
with conventional gait training and to investigate its impact on gait performance in chronic stroke
patients.[…]
[WEB] Optimized tDCS in Stroke Rehabilitation: A Comparative Study and New Insights
Posted by Kostas Pantremenos in REHABILITATION, tDCS/rTMS on February 5, 2024

Stroke rehabilitation is a critical aspect of healthcare that aids in restoring independence and improving quality of life for stroke survivors. Innovative neurorehabilitation strategies, like transcranial direct current stimulation (tDCS), have been increasingly recognized for their potential in enhancing motor recovery after stroke. A recent study has shed light on the differences between optimized and conventional tDCS approaches. The findings suggest that optimized tDCS may have a greater potential for improving motor rehabilitation in stroke patients.
Unraveling the Differences: Optimized and Conventional tDCS
The study revealed that the electrode positions of optimized tDCS differed significantly from those of conventional tDCS. Furthermore, the electric field intensity in the brain was found to be higher with optimized tDCS. Importantly, the electrode positions in the optimized method were influenced by the location of the stroke lesion. Larger differences were observed in patients with cortical lesions, emphasizing that personalized montages in tDCS are critical for effective stimulation.
Stroke Lesion Conductivity and Electric Field Distribution
However, the study was not without limitations. Uncertainties about stroke lesion conductivity and the presentation of the electric field distribution remained. These aspects require further research and clarification to fully understand the potential of optimized tDCS in stroke rehabilitation.
Other Studies on Brain Stimulation
Several other studies have explored the effectiveness of various forms of brain stimulation in stroke rehabilitation. For instance, a model-based study focused on optimizing non-invasive brain electrical stimulation for Parkinson’s disease. It suggested that Transcranial Electrical Stimulation (TES) could alter the firing pattern of cells in the Basal Ganglia and reduce Parkinsonian behavior.
A systematic review with a meta-analysis estimated the effectiveness of combining robot-assisted therapy (RAT) and tDCS in motor recovery of the upper extremities after stroke. However, it found that the addition of tDCS to RAT produced a negligible additional benefit on the effects of upper limb function, hand dexterity, spasticity, and activity.
Exploring Other Forms of Brain Stimulation
Theta burst stimulation (TBS) and transcranial alternating current stimulation (tACS) have also been explored. A systematic review of existing evidence from randomized controlled trials found that TBS might yield more pronounced and long-lasting after-effects than conventional patterns of repetitive transcranial magnetic stimulation (rTMS). A study protocol for a randomized controlled trial is examining the effectiveness and brain mechanisms of multi-target tACS on motor learning in stroke patients.
Future Directions
While the potential of optimized tDCS and other forms of brain stimulation in stroke rehabilitation is evident, more research is needed to fully understand their benefits and limitations. A randomized clinical trial is currently underway to further explore the effectiveness of optimized tDCS in improving upper extremity function in stroke patients. These findings will undoubtedly contribute to the evolving landscape of stroke rehabilitation, offering new insights and potential therapeutic avenues for clinicians and patients alike.
[Abstract] Transcranial Direct Current Stimulation and Brain–Computer Interfaces for Improving Post-Stroke Recovery: A Systematic Review and Meta-Analysis
Posted by Kostas Pantremenos in REHABILITATION, tDCS/rTMS on November 8, 2023
Abstract
Objective
This study aimed to evaluate the effectiveness of transcranial direct current stimulation associated with brain–computer interface in stroke patients.
Data sources
The PubMed, Central, PEDro, Web of Science, SCOPUS, PsycINFO Ovid, CINAHL EBSCO, EMBASE, and ScienceDirect databases were searched from inception to April 2023 for randomized controlled studies reporting the effects of active transcranial direct current stimulation associated with brain–computer interface to a transcranial direct current stimulation sham associated with brain–computer interface condition on the outcome measure (motor performance and functional independence).
Review methods
We searched for full-text articles which had investigated the effect of transcranial direct current stimulation associated with brain–computer interface on motor performance in the upper extremities in stroke patients. The standardized mean differences derived from the change in scores between pretreatment and post-treatment were adopted as the effect size measure, with a 95% confidence interval. Possible sources of heterogeneity were analyzed by performing subgroup analyses in order to examine the moderating effects for one variable: the level of injury severity.
Results
Nine studies were included in the qualitative synthesis and the meta-analysis. The findings of the conducted analyses indicated there is not enough evidence to suggest that active transcranial direct current stimulation associated with brain–computer interface is more efficient in motor performance and functional independence when compared to sham transcranial direct current stimulation associated with brain–computer interface or brain–computer interface alone. In addition, the quality of evidence was rated very low. A subgroup analysis was performed for the motor performance outcome considering the injury severity level.
Conclusion
We found evidence that transcranial direct current stimulation associated with brain–computer interface was not more beneficial than sham transcranial direct current stimulation associated with brain–computer interface or brain–computer interface alone.
Get full access to this article
View all access and purchase options for this article.
References
1. Boden-Albala B, Appleton N, Schram B. Stroke epidemiology and prevention. In: Wilson R, Raghavan P (eds) Stroke rehabilitation. St. Louis, MO: Elsevier, 2019, pp.1–21.
2. Lee JJ. Lower limb impairments after stroke. In: Wilson R, Raghavan P (eds) Stroke rehabilitation. St. Louis, MO: Elsevier, 2019, pp.123–132.
3. Feng W, et al. Trans cranial direct current stimulation for poststroke motor recovery: challenges and opportunities. PM&R 2018; 10: S157–S164.
4. Elsner B, Kugler J, Pohl M, et al. Transcranial direct current stimulation (tDCS) for improving activities of daily living, and physical and cognitive functioning, in people after stroke. Cochrane Database Syst Rev 2016; 3: 1–168.
5. Fregni F, et al. Evidence-based guidelines and secondary meta-analysis for the use of transcranial direct current stimulation in neurological and psychiatric disorders. Int J Neuropsychopharmacol 2021; 24: 256–313.
6. Shih JJ, Krusienski DJ, Wolpaw JR. Brain-computer interfaces in medicine. Mayo Clin Proc 2012; 87: 268–279.
7. van Dokkum LEH, Ward T, Laffont I. Brain computer interfaces for neurorehabilitation—its current status as a rehabilitation strategy post-stroke. Ann Phys Rehabil Med 2015; 58: 3–8.
8. Danzl M, Chelette K, Lee K, et al. Brain stimulation paired with novel locomotor training with robotic gait orthosis in chronic stroke: a feasibility study. NeuroRehabilitation 2013; 33: 67–76.
9. Hesse S, et al. Combined transcranial direct current stimulation and robot-assisted arm training in subacute stroke patients: an exploratory, randomized multicenter trial. Neurorehabil Neural Repair 2011; 25: 838–846.
10. Cheng HJ, et al. Task-related brain functional network reconfigurations relate to motor recovery in chronic subcortical stroke. Sci Rep 2021; 11: 1–12.
11. Ang KK, et al. Facilitating effects of transcranial direct current stimulation on motor imagery brain-computer interface with robotic feedback for stroke rehabilitation. Arch Phys Med Rehabil 2015; 96: S79–S87.
12. Chew E, et al. Using transcranial direct current stimulation to augment the effect of motor imagery-assisted brain-computer interface training in chronic stroke patients—cortical reorganization considerations. Front Neurol 2020; 11: 948.
13. Hong X, et al. Brain plasticity following MI-BCI training combined with tDCS in a randomized trial in chronic subcortical stroke subjects: a preliminary study. Sci Rep 2017; 7: 9222.
14. Hu M, et al. Brain functional changes in stroke following rehabilitation using brain-computer interface-assisted motor imagery with and without tDCS: a pilot study. Front Hum Neurosci 2021; 15: 692304.
15. Mane R, et al. Prognostic and monitory EEG-biomarkers for BCI upper-limb stroke rehabilitation. IEEE Trans Neural Syst Rehabil Eng 2019; 27: 1654–1664.
16. Kasashima-Shindo Y, et al. Brain-computer interface training combined with transcranial direct current stimulation in patients with chronic severe hemiparesis: proof of concept study. J Rehabil Med 2015; 47: 318–324.
17. Straudi S, et al. tDCS and robotics on upper limb stroke rehabilitation: effect modification by stroke duration and type of stroke. Biomed Res Int 2016; 2016: 5068127.
18. Comino-Suárez N, et al. Transcranial direct current stimulation combined with robotic therapy for upper and lower limb function after stroke: a systematic review and meta-analysis of randomized control trials. J Neuroeng Rehabil 2021; 18: 148.
19. Guerra A, López-Alonso V, Cheeran B, et al. Solutions for managing variability in non-invasive brain stimulation studies. Neurosci Lett 2017; 719: 133332.
20. Guerra A, López-Alonso V, Cheeran B,. et al. Variability in non-invasive brain stimulation studies: reasons and results. Neurosci Lett 2020; 719: 133330.
21. Ang KK, Guan C. Brain-computer interface in stroke rehabilitation. J Comput Sci Eng 2013; 7: 139–146.
22. Bai Z, Fong KNK, Zhang JJ, et al. Immediate and long-term effects of BCI-based rehabilitation of the upper extremity after stroke: a systematic review and meta-analysis. J Neuroeng Rehabil 2020; 17: 1.
[ARTICLE] Do Higher Transcranial Direct Current Stimulation Doses Lead to Greater Gains in Upper Limb Motor Function in Post-Stroke Patients? – Full Text
Posted by Kostas Pantremenos in Paretic Hand, tDCS/rTMS on August 12, 2023
Abstract
Objective: To investigate whether a higher number of transcranial direct current stimulation (tDCS) sessions results in a greater improvement in upper limb function in chronic post-stroke patients. Materials and methods: A randomized, sham-controlled, double-blind clinical trial was conducted in 57 chronic post-stroke patients (≥ 3 months after their injuries). The patients were allocated to receive sessions of tDCS combined with physiotherapy and divided into three groups (anodal, cathodal, and sham). The Fugl-Meyer Assessment of Upper Extremity (FMA-UE) was used to assess the sensorimotor impairment of the patients’ upper limbs before (baseline) and after five and ten sessions. The percentage of patients who achieved a clinically significant improvement (> five points on the FMA-UE) was also analyzed. Results: The FMA-UE score increased after five and ten sessions in both the anodal and cathodal tDCS groups, respectively, compared to the baseline. However, in the sham group, the FMA-UE score increased only after ten sessions. When compared to the sham group, the mean difference from the baseline after five sessions was higher in the anodal tDCS group. The percentage of individuals who achieved greater clinical improvement was higher in the stimulation groups than in the sham group and after ten sessions when compared to five sessions. Conclusions: Our results suggest that five tDCS sessions are sufficient to augment the effect of standard physiotherapy on upper limb function recovery in chronic post-stroke patients, and ten sessions resulted in greater gains.
1. Introduction
Stroke is one of the leading causes of physical disability among adults worldwide [1], with approximately 77% of survivors having chronic sensorimotor deficits that affect functional independence [2]. Often after a stroke upper extremity motor function is impaired, affecting patients’ daily living activities and quality of life [3]. Limb motor function is spontaneously recovered within six months [4,5], but rehabilitation can improve motor function even in the chronic phase [6]. However, motor function recovery through rehabilitation can be time-consuming and depends on plasticity [7,8]. Therefore, there is growing interest in tools that promote plasticity to enhance rehabilitation outcomes [9].
Transcranial direct current stimulation (tDCS) is a potential tool for increasing and accelerating cerebral cortex reorganization and facilitating motor learning by modulating cortical excitability [10]. After a stroke, tDCS promotes motor learning, known as priming, and can be used before or during motor training [11,12] to maximize upper limb motor gains [13]. Usually, anodal tDCS is applied to the motor cortex of the lesioned hemisphere to increase neuronal excitability, and cathodal tDCS in the non-lesioned hemisphere to decrease neuronal excitability [10,14,15,16,17].
Increasing evidence points to tDCS as an adjunctive treatment in post-stroke motor rehabilitation [18,19]. Despite promising evidence suggesting that tDCS potentializes stroke recovery [20,21,22], tDCS is not a one-size-fits-all treatment [23]. Indeed, studies have highlighted the variability in the effects of tDCS in stroke patients [24,25,26]. Understanding the factors affecting individual responses to electrical stimulation is crucial for identifying the optimal tDCS protocol to promote functional recovery after a stroke.
Several factors can influence the achievement of the best motor response with tDCS post-stroke, and questions still remain regarding the ideal dose of the number of sessions [23]. Indeed, randomized controlled trials (RCTs) that involved the use of intervention protocols with tDCS achieved significant improvements in upper limb (UL) motor function but with a large variation in the number of sessions [15,27,28,29,30,31,32,33,34,35]. These studies reported similar outcomes with five [15], ten [27,28,29,30,31], twelve [33,34], eighteen [34], and thirty-six sessions with the stimulation protocol [35], making it difficult to identify dose–response relationships. As such, our understanding of dose responses is limited.
To the best of our knowledge, no randomized clinical trial has investigated whether higher doses of tDCS produce greater clinical improvement in upper limb motor function. The dose of a non-pharmaceutical intervention is unclear, and inconsistent terminology can incorporate multiple dose dimensions, such as frequency, intensity, duration, and intervention length [36]. Here, the term dose is used to denote the dose dimension of the number of intervention sessions over time. Thus, we conducted a two-dose trial to compare the effect of the minimal number of sessions in studies with multiple sessions (five sessions) and the most common number of sessions among the studies (ten sessions). We hypothesized that higher doses of tDCS combined with physiotherapy would result in greater sensorimotor recovery of the upper limbs in chronic post-stroke patients. Additionally, we hypothesized a different dose–response relationship between the types of stimulation (cathodal vs. anodal tDCS). […]
Continue—–> https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9859554/

Percentage of individuals who achieved no change (below five points on Fugl-Meyer Assessment of Upper Extremity), much improvement (above five points), and very much improvement (above ten points) in upper limb motor function after five and 10 sessions of anodal (gray circles), cathodal (orange circles), and sham (blue circles) transcranial direct current stimulation (tDCS).
[ARTICLE] Transcranial direct current stimulation with virtual reality versus virtual reality alone for upper extremity rehabilitation in stroke: A meta-analysis – Full Text
Posted by Kostas Pantremenos in Paretic Hand, tDCS/rTMS, Virtual reality rehabilitation on January 8, 2023
Abstract
Background
Stroke is one of the most prevalent diseases. Motor impairment in patients with stroke frequently affects the upper extremities. Several randomized clinical trials (RCTs) have tried to prove whether or not the combination of transcranial direct current stimulation (tDCS) with virtual reality (VR) is superior to VR alone for upper extremity rehabilitation.
Methods
We searched Embase, MEDLINE, the Cochrane Library database, and Clinicaltrials.gov for relevant RCTs published before June 10, 2022. The results were analyzed by using standard mean differences (SMD) and 95% confidence intervals (95% CI).
Results
We pooled 120 patients from 4 RCTs. There were no significant improvements in the Fugl-Meyer Upper Extremity scale (SMD = 0.51; 95% CI, −0.04 to 1.06), the Box and Block Test (SMD = 0.42; 95% CI, −0.02 to 0.86), and the Modified Ashworth Scale after the combined treatment of tDCS and VR. But tDCS combined with VR could enhance the Barthel Index scores in patients with stroke compared to VR alone (SMD = 0.49; 95% CI, 0.04 to 0.94).
Conclusions
The combination of tDCS and VR can improve the quality of daily living in patients with stroke. No more satisfactory efficacy has been demonstrated in terms of upper extremity function. However, we observe a distinct trend toward significance in some outcomes.
1. Introduction
Stroke is a cerebrovascular disease with high morbidity, disability, and mortality rate. Stroke has become the second common cause of death globally and in several countries stroke has become the most common cause of death, according to a study spanning nearly 40 years [1]. Even if they survive, 80% of patients have varying degrees of neurological deficits throughout their lives [2] and the loss of high disability-adjusted life years leaves patients suffering greatly [3]. The incidence of stroke has increased by 68% in recent decades [4]. Therefore, not only the prevention and timely treatment of stroke is extremely important, but also post-stroke rehabilitation.
One of the most common symptoms of stroke is unilateral hemiparesis [4], which particularly presents with upper and lower extremity dysfunction. Hence, rehabilitation becomes urgent and essential when aggressive and effective treatment fails to accomplish. More and more rehabilitation approaches are being used for patients with post-stroke [5]. Virtual reality (VR) can communicate the virtual world with reality, allowing people to experience the virtual world more realistically. Initially, VR was used to enhance the gaming experience. With the development of technology, VR had the characteristics of experiential learning, augmented feedback, observational learning, and goal-oriented, so it was encouraging to see that some research used VR in the medical industry with satisfactory results [6, 7, 8, 9, 10, 11, 12]. In the case of the upper extremity, only 11.6% of the patients are able to regain full function at 6 months post-stroke [13]. Combining with VR is an easier way for patients with upper extremity hemiplegia to benefit from rehabilitation compared to conventional physical rehabilitation [12]. However, some limitations occur in the use of VR [14].
Non-invasive brain stimulation can modulate cortical excitability. Transcranial direct current stimulation (tDCS) as a type of non-invasive brain stimulation is gradually applied in clinical treatment because of lower cost, easier operation, and better security [15]. A meta-analysis has demonstrated that tDCS improves motor performance in patients recovering from chronic stroke or mild to moderate stroke [16]. In addition, Kim et al. found the combined effect and a stronger short-term corticospinal facilitation of tDCS with VR [17]. Llorens et al. also proved that the combination of tDCS and VR was significantly more efficacy than conventional physical therapy [18]. These trials investigated the possibility that patients could achieve a better long-term prognosis by receiving both noninvasive brain stimulation with tDCS and guided training with VR. Some articles suggested that upper extremity function in patients with stroke could benefit more from the combination of tDCS and VR compared to VR alone [19, 20, 21]. However, no study has specifically and systematically evaluated the efficacies of the combination of tDCS and VR versus VR alone for upper extremity training until now. Therefore, we conduct this meta-analysis and systematic review to assess whether the combination of tDCS and VR is better than VR alone. […]
[ARTICLE] MRI-Based Personalized Transcranial Direct Current Stimulation to Enhance the Upper Limb Function in Patients with Stroke: Study Protocol for a Double-Blind Randomized Controlled Trial – Full Text
Posted by Kostas Pantremenos in Paretic Hand, Radiology/Imaging technology, tDCS/rTMS on December 11, 2022
Abstract
Transcranial direct current stimulation (tDCS) has been shown to have the potential to improve the motor recovery of the affected upper limbs in patients with stroke, and recently, several optimized tDCS methods have been proposed to magnify its effectiveness. This study aims to determine the effectiveness of personalized tDCS using brain MRI-based electrical field simulation and optimization, to enhance motor recovery of the upper limbs in the patients. This trial is a double-blind, randomized controlled trial in the subacute to chronic rehabilitation phase. Forty-two adult stroke patients with unilateral upper limb involvement will be randomly allocated to three groups: (1) personalized tDCS with MRI-based electrical field simulation and optimized stimulation, (2) conventional tDCS with bihemispheric stimulation of the primary motor cortex, and (3) sham tDCS. All three groups will undergo 10 intervention sessions with 30 min of 2-mA intensity stimulation, during a regular upper limb rehabilitation program over two weeks. The primary outcome measure for the motor recovery of the upper limb impairment is the Fugl–Meyer assessment for the upper extremity score at the end of the intervention, and the secondary measures include changes in the motor evoked potentials, the frequency power and coherence of the electroencephalography, performance in activities of daily living, and adverse events with a 1-month follow-up assessment. The primary outcome will be analyzed on the intention-to-treat principle. There is a paucity of studies regarding the effectiveness of personalized and optimized tDCS that considers individual brain lesions and electrical field characteristics in the real world. No feasibility or pivotal studies have been performed in stroke patients using brain MRI, to determine a lesion-specific tDCS simulation and optimization that considers obstacles in the segmentation and analysis of the affected brain tissue, such as ischemic and hemorrhagic lesions. This trial will contribute to addressing the effectiveness and safety of personalized tDCS, using brain MRI-based electrical field simulation and optimization, to enhance the motor recovery of the upper limbs in patients with stroke.
1. Introduction
Many patients complain of upper limb paralysis, which remains, in many cases, as a disability that interferes with the independence of daily living [1]. These motor impairments of the upper limbs would be known to relate to the corticospinal tract (CST) integrity, originating from the primary motor cortex [2,3]. In addition, the cerebellar afferent and efferent tracts also contribute to the motor function of the upper limbs [4,5]. Combining this knowledge for the anatomical basis of the upper limb motor function, the primary motor cortex and CST are the most critical structures for the motor function of the upper limbs. Thus, previous studies for noninvasive neuromodulation via repetitive transcranial magnetic stimulation (rTMS) or transcranial direct current stimulation (tDCS), have targeted the ipsilesional primary motor cortex for enhancing the motor function of the upper limbs [6,7,8].
Proper treatment and effective recovery in patients with a stroke are important issues for the patients themselves, their families, neurologists, therapists and physiotherapists. Many stroke survivors have ongoing impairments of the upper limbs [9]. Recently, noninvasive brain stimulation has been introduced to reduce the impairments of the upper limbs [8,10,11]. Among the various types of noninvasive brain stimulation, transcranial direct current stimulation (tDCS) is being actively studied [12,13]. The tDCS has recently received the most attention because of its safety, convenience, and portability [12,14,15] A constant, low-intensity current (1 to 2 mA) is passed through two electrodes placed over the head and modulates the neuronal activity [16,17]. The widespread opinion is that anodal stimulation increases the cortical excitability, whereas cathodal stimulation reduces the cortical excitability (by hyperpolarization and depolarization, respectively) [18].
The level of evidence for tDCS is still low because a standardized protocol has not been fully established [19]. Particularly, this is because the results of studies that tried to verify the tDCS effects were inconsistent [20], and a main factor was the large variation in the treatment effect between individuals, even when the same protocol was applied [21]. High levels of interindividual variations in the tDCS effects are thought to be because the low-intensity current transmitted through the scalp is influenced by the brain structure, such as the characteristics of brain anatomy [22]. As a result, these inconsistent findings have been taken as a lack of evidence for its effectiveness [23,24]. Although a 10–20 EEG guide was introduced to apply tDCS electrodes in a manner that considers the variation in individuals’ head geometry, the guide did not provide an exact match for the patients’ brains in clinical neurorehabilitation settings.
Recently, some studies have attempted to define the optimized stimulation parameters and to reveal the mechanisms of interaction between the electric field (E-field) induced by tDCS and the cortical neurons and their therapeutic effects [22]. The lack of an analytical and technical pipeline enabling the optimization of stimulation parameters across individuals is a factor that interferes with the accumulation of evidence. Optimization methods requiring the analysis of individual MR images to make a brain model and the calculation of E-fields induced by tDCS, is very labor intensive, and there is an inconvenience in using multiple types of research software. These reasons have blocked the application of tDCS with the calculated E-fields in the clinical setting. Thus, we recently developed a method that analyzes the magnetic resonance image of an individual, to generate a brain model and then calculates the magnitude of the electric field generated by tDCS, through the analysis of the brain structure, in a single software package [25]. To maximize the effect of tDCS, not only the individual brain structures but also the stroke lesions must be considered [26], so the software was developed to include methods that could segment the stroke lesions and calculate the conductivity of the stroke lesions. One previous study reported that the optimized electrode montages which applied MRI-based individual head models for tDCS, could yield the maximal stimulation target, compared to conventional montages [27]. However, the study has no results about the clinical effect.
Taking into account the mentioned limitations of the conventional tDCS method and the results of a previous study, our ongoing development of optimized software, and current clinical demands, we hypothesized that MRI-based optimized personalized tDCS would be better than conventional tDCS, to treat patients with a stroke for improving the upper limb function. Thus, we will investigate the efficacy of the MRI-based optimized personalized tDCS for improving the upper limb function in patients with a subacute and chronic stroke via a multicenter prospective randomized double-blinded controlled trial. We present the rationale and precise methods from our planned trial: MRI-based personalized transcranial direct current stimulation to enhance the upper limb function in patients with a stroke: A randomized double-blinded controlled trial. The proposed protocol seeks to establish the benefits from personalized anodal tDCS with two control groups, a sham control group and a conventional anodal tDCS group, to prove the treatment efficacy of the upper limb motor function in patients with a stroke. […]

[Abstract] Noninvasive brain stimulation for cognitive rehabilitation following traumatic brain injury: a systematic review
Posted by Kostas Pantremenos in Cognitive Rehabilitation, TBI, tDCS/rTMS on December 6, 2022
Abstract
Traumatic brain injury (TBI) can cause numerous cognitive deficits. These deficits are associated with disability and reduction in quality of life. Noninvasive brain stimulation (NIBS) provides excitatory or inhibitory stimuli to the cerebral cortex. This review aimed to examine the effectiveness of NIBS (i.e., rTMS and tDCS) on cognitive functions in patients with TBI. PubMed, SCOPUS, PEDro, CINAHL, MEDLINE, REHABDATA, and Web of Science were searched from inception to May 2021. The risk of bias in the randomized controlled trials was assessed using the Cochrane Collaboration’s instrument. The Physiotherapy Evidence Database (PEDro) scale was applied to evaluate the risk of bias in the non-randomized controlled trials. Ten studies met our inclusion criteria. Six studies used repetitive Transcranial Magnetic Stimulation (rTMS), and four used transcranial Direct Current Stimulation (tDCS) as cognitive rehabilitation interventions. The results showed heterogenous evidence for the effects of rTMS and tDCS on cognitive function outcomes in individuals with TBI. The evidence for the effects of NIBS on cognition following TBI was limited. TDCS and rTMS are safe and well-tolerated interventions post-TBI. The optimal stimulation sites and stimulation parameters remain unknown. Combining NIBS with traditional rehabilitation interventions may contribute to greater enhancements in cognitive functions post-TBI.
Similar articles
- The Effect of Non-Invasive Brain Stimulation (NIBS) on Executive Functioning, Attention and Memory in Rehabilitation Patients with Traumatic Brain Injury: A Systematic Review.Hara T, Shanmugalingam A, McIntyre A, Burhan AM.Diagnostics (Basel). 2021 Mar 31;11(4):627. doi: 10.3390/diagnostics11040627.PMID: 33807188 Free PMC article. Review.
- The Effect of Non-Invasive Brain Stimulation (NIBS) on Attention and Memory Function in Stroke Rehabilitation Patients: A Systematic Review and Meta-Analysis.Hara T, Shanmugalingam A, McIntyre A, Burhan AM.Diagnostics (Basel). 2021 Feb 3;11(2):227. doi: 10.3390/diagnostics11020227.PMID: 33546266 Free PMC article. Review.
- Noninvasive Brain Stimulation for Rehabilitation of Pediatric Motor Disorders Following Brain Injury: Systematic Review of Randomized Controlled Trials.Elbanna ST, Elshennawy S, Ayad MN.Arch Phys Med Rehabil. 2019 Oct;100(10):1945-1963. doi: 10.1016/j.apmr.2019.04.009. Epub 2019 May 10.PMID: 31078616
- Effects of Noninvasive Brain Stimulation (NIBS) on Cognitive Impairment in Mild Cognitive Impairment and Alzheimer Disease: A Meta-analysis.Wang T, Guo Z, Du Y, Xiong M, Yang Z, Ren L, He L, Jiang Y, McClure MA, Mu Q.Alzheimer Dis Assoc Disord. 2021 Jul-Sep 01;35(3):278-288. doi: 10.1097/WAD.0000000000000464.PMID: 34432674
- Non-invasive brain stimulation techniques for chronic pain.O’Connell NE, Marston L, Spencer S, DeSouza LH, Wand BM.Cochrane Database Syst Rev. 2018 Mar 16;3(3):CD008208. doi: 10.1002/14651858.CD008208.pub4.PMID: 29547226 Free PMC article. Updated. Review.
[WEB] REMOTE Supervise – Soterix Medical
Posted by Kostas Pantremenos in tDCS/rTMS on November 20, 2022
Remote Neuromodulation
Deployed transcranial electrical stimulation, without compromise.

Soterix Medical REMOTE Neuromodulation is the only system with device, accessories, and software designed for deployed use. Safe transcranial Electrical Stimulation requires advanced systems designed for consistency and control. REMOTE Neuromodulation is the only system designed from the ground up to allow translation of clinical tES, including tDCS protocols, to diverse deployed environments, while maintaining medical standards.
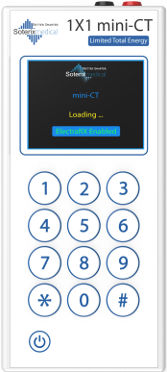
mini-CT device
Fail-safe and intelligent hand-held stimulators designed specifically for deployed use. Deliver only the right dose at the right time, with onboard compliance recording and ElectraRx compatibility Learn more…
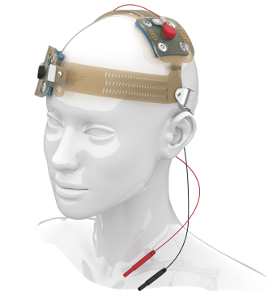

SNAP Headgear accessories
The only head-gear that is simple to self-apply and guarantees accurate electrode placement. The only pre-saturated snap electrodes for tES in deployed environments
Learn more…
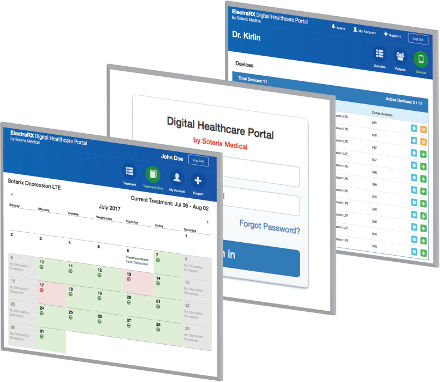
ElectraRx software
ElectraRx integrates complete screening and assessments, tDCS dose management, and online digital therapeutics all in one place that can be accessed from anywhere
Learn more…
Soterix Medical REMOTE Neuromodulation systems are governed by three design principles:

Supervised Care
REMOTE Neuromodulation starts and ends with a professional caregiver. Dose control, limits, and remote monitoring are ensured thought unique systems and controls.
Right Equipment
Accessories + intelligent hardware equipped with our proprietary LTE technology that are designed for safe and consistent application in deployed environment.

Trial Customizations
Soterix Medical is the technology leader in noninvasive electrical stimulation and has developed advanced task-specific neuromodulation devices.
Soterix Medical REMOTE
Neuromodulation systems are uniquely validated
Soterix Medical hardware and accessories for remote use are the only equipment designed and validated for that purpose. Other systems designed for use by professionals and medical and research centers cannot be rigged for home use.
Soterix Medical REMOTE is supported by over a dozen clinical trials including the demonstration of tolerability, reproducibility, and compliance.

- Feasibility of remote transcranial direct current stimulation for pediatric cerebral palsy during the COVID-19 pandemic. Brain Stimul. 2020.
- A double-blind sham-controlled phase 1 clinical trial of tDCS of the dorsolateral prefrontal cortex in cocaine inpatients: Craving, sleepiness, and contemplation to change. Neurosci. 2021
- Effects of combined theta burst stimulation and transcranial direct current stimulation of the dorsolateral prefrontal cortex on stress. International Federation of Clinical Neurophysiology 2021
- Transcranial direct current stimulation of dorsolateral prefrontal cortex improves dual-task gait performance in patients with Parkinson’s disease: A double blind, sham-controlled study. Gait & posture 2021
See all publications
For more visit site
[WEB] Home-Based Transcranial Stimulation Succeeds for Major Depression
Posted by Kostas Pantremenos in Depression, tDCS/rTMS, Tele/Home Rehabilitation on October 19, 2022
Home-based transcranial direct current stimulation with real-time supervision significantly improved clinical symptoms of major depressive disorder, based on data from 26 individuals.
Major depressive disorder (MDD) remains a leading cause of disability and a significant predictor of suicide worldwide, Rachel D. Woodham, PhD, of the University of East London and colleagues wrote.
Transcranial direct current stimulation (tDCS) has demonstrated effectiveness as a noninvasive therapy for MDD, but requires frequent sessions, and repeat visits to treatment centers are a barrier for many patients, they noted. The tDCS procedure involves delivery of a weak direct electric current via placement of electrodes, usually with the anode over the left dorsolateral prefrontal cortex and the cathode over the right dorsolateral prefrontal cortex, suborbital, or frontotemporal region.
“The current changes neuronal membrane potential and facilitates discharge,” but “in contrast to rTMS and ECT, tDCS does not directly trigger an action potential,” the researchers wrote. The most common side effects reported with tDCS are tingling, itching, burning sensation, skin redness or headache.
The researchers proposed that tDCS could be provided at home under real-time remote supervision.
In an open-label feasibility study published in the Journal of Psychiatric Research, they recruited 26 adults with MDD in current depressive episodes of moderate to severe severity. In addition to maintaining their current treatment regimens of medication, psychotherapy, or cognitive behavioral therapy, participants used tDCS at home in 30-minute sessions, for a total of 21 sessions over 6 weeks. A researcher was present in person or on a real-time video call for each at-home session.
The primary outcome of Hamilton Rating Scale for Depression (HAMD) score improved significantly, from a mean of 19.12 at baseline to 5.33 after 6 weeks. At 3 months, the mean HAMD score was 5.65, and 78.2% of patients met the criteria for clinical remission (HAMD score less than 9). At 6 months, patients maintained this improvement, with a mean HAMD score of 5.43 and 73.9% of the participants in clinical remission. The majority of participants (24 of 26) completed the full 6-week treatment.
Clinical assessments were conducted at baseline, at the end of the 6-week treatment period, at 3 months, and at 6 months, and included not only the HAMD, but also the Hamilton Anxiety Rating Scale (HAMA), Sheehan Disability Scale (SDS), Patient Health Questionnaire–9 (PHQ-9), and Young Mania Rating Scale. All participants showed significant improvements in HAMA, SDS, and PHQ-9 scores from baseline that endured from the end of the treatment period to the 6 months’ follow-up.
The tDCS involved a bilateral frontal montage, F3 anode, F4 cathode, 2mA, and two different devices were used.
All participants reported the acceptability of at-home tDCS as either “very acceptable” or “quite acceptable.”
The results were limited by the open-label feasibility design and lack of a sham control treatment; therefore, the findings of efficacy are preliminary, the researchers emphasized. “Having real-time supervision for each session likely contributed to symptom improvement.”
However, the results support the feasibility of at-home tDCS to improve outcomes both short- and long-term in patients with moderate to severe MDD, the researchers said. Larger, sham-controlled trials are needed to show efficacy, and additional assessment of feasibility should include the use of app-based devices, which may be more feasible for individuals with lower socioeconomic status.
The study received no outside funding. The study was supported by the Rosetrees Trust. The researchers had no financial conflicts to disclose.
This article originally appeared on MDedge.com, part of the Medscape Professional Network.
[REVIEW] Cognitive Fatigability Interventions in Neurological Conditions: A Systematic Review – Full Text
Posted by Kostas Pantremenos in Fatigue on October 3, 2022
Abstract
Introduction
Although fatigue is a well-studied concept in neurological disease, cognitive fatigability (CF) is less understood. While most studies measure fatigue using subjective self-report, fewer have measured CF objectively. Given the negative impact of CF on quality-of-life, there is a need for targeted interventions. The objective of this review was to determine which procedural, behavioural and pharmacological treatments for objectively measured CF are available to people living with neurological conditions.
Methods
In accordance with the PRISMA guidelines, systematic searches for randomized control trials (RCTs), case-controlled studies and case reports/series were conducted across the Ovid Medline, PsycInfo, EMBASE and Cochrane Library databases. English-language articles published between 1980 and February 2019 were considered for eligibility. Included were those that objectively measured CF in individuals with neurological disease/disorder/dysfunction between the ages of 18 and 65 years. Studies were reviewed using a modified Cochrane Data Extraction Template. Risk of bias was assessed using the Cochrane Risk of Bias tool. The review process was facilitated using Covidence software (www.covidence.org). Two authors reviewed articles independently, with a third resolving conflicts regarding article inclusion.
Results
The search identified 450 records. After duplicates were removed and remaining titles/abstracts were screened for eligibility, 28 full-text articles were assessed, and two studies were included in the qualitative synthesis. Studies were a priori divided into those with pharmacological, procedural or behavioural interventions. Two studies met eligibility criteria; both of these included participants with multiple sclerosis. One study utilized a procedural intervention (i.e. transcranial direct current stimulation), while the other utilized a pharmacological intervention (i.e. fampridine-SR). Studies were evaluated for risk of bias, and evidence from both eligible studies was discussed.
Conclusion
Despite the positive results of the procedural intervention, the paucity of eligible studies and the nascent nature of the field suggests that more studies are required before firm conclusions can be drawn regarding the amenability of CF to treatment.
Introduction
Although fatigue is a well-studied concept in neurological disease, cognitive fatigability (CF) is less understood. While most studies measure fatigue using subjective self-report, fewer have measured CF objectively. Given the negative impact of CF on quality-of-life, interventions are beginning to be introduced to address this concern, but the field is in its infancy and there is little agreement on treatment approach. There is a need for a thorough review of the literature to classify the current state of the field and to identify needs moving forward.
What is meant by the term fatigue varies throughout the literature. Definitions include a state of reduced capacity for work following a period of mental or physical activity [1], a feeling of physical tiredness and lack of energy that is distinct from sadness or weakness [2], extreme tiredness with the feeling that one needs to rest [3], a feeling of lack of energy, weariness and aversion to effort [4], among other definitions [5]. Fatigue has largely been regarded as a subjective experience unique to the individual, and thus it is typically measured by self-report. A number of questionnaires have been developed to that end, with some attempting to quantify fatigue and others evaluating the impact of fatigue on daily functioning. These include: the Fatigue Severity Scale [6], the Fatigue Impact Scale [7] (and the modified version [8]), the Neurological Fatigue Index [9], the Mental Fatigue Scale [10], the Fatigue Assessment Scale [11], the Multidimensional Fatigue Inventory [12] and the Fatigue Scale for Motor and Cognitive Functions [13, 14].
Fatigue is a common symptom in a variety of neurological diseases. It is the most commonly reported complaint in those with multiple sclerosis (MS), occurring in up to 90% of individuals affected with this disease [15, 16], with negative impacts on quality-of-life [17], self-esteem [18] and employability [19, 20]. It is present in 50% of those with traumatic brain injury (TBI) [21], and affected individuals often endorse fatigue as one of the most challenging and distressing long-lasting symptoms [22, 23]. Fatigue provides a unique contribution to TBI-related disability after controlling for injury severity, executive functions and depression [24]. It occurs in 25–85% of stroke survivors [25] and has a significant impact on the employability of the individual and society as a whole given the economic impacts [26]. A consensus report from UK stroke survivors, caregivers and health professionals noted that fatigue was in the top ten research priorities due to its debilitating consequences and the incomplete understanding of the mechanisms and treatments [27]. Negative impacts of fatigue have also been documented in those with Parkinson’s disease [28, 29], amyotropic lateral sclerosis [30] and primary brain tumour [31]. […]
