In the United States more than 700,000 people suffer a stroke each year, and approximately two-thirds of these individuals survive and require rehabilitation. The goals of rehabilitation are to help survivors become as independent as possible and to attain the best possible quality of life. Even though rehabilitation does not “cure” the effects of stroke in that it does not reverse brain damage, rehabilitation can substantially help people achieve the best possible long-term outcome.
What is post-stroke rehabilitation?
Rehabilitation helps stroke survivors relearn skills that are lost when part of the brain is damaged. For example, these skills can include coordinating leg movements in order to walk or carrying out the steps involved in any complex activity. Rehabilitation also teaches survivors new ways of performing tasks to circumvent or compensate for any residual disabilities. Individuals may need to learn how to bathe and dress using only one hand, or how to communicate effectively when their ability to use language has been compromised. There is a strong consensus among rehabilitation experts that the most important element in any rehabilitation program is carefully directed,well-focused, repetitive practice—the same kind of practice used by all people when they learn a new skill, such as playing the piano or pitching a baseball.
Rehabilitative therapy begins in the acute-care hospital after the person’s overall condition has been stabilized, often within 24 to 48 hours after the stroke. The first steps involve promoting independent movement because many individuals are paralyzed or seriously weakened. Patients are prompted to change positions frequently while lying in bed and to engage in passive or active range of motion exercises to strengthen their stroke-impaired limbs. (“Passive” range-of-motion exercises are those in which the therapist actively helps the patient move a limb repeatedly, whereas “active” exercises are performed by the patient with no physical assistance from the therapist.) Depending on many factors—including the extent of the initial injury—patients may progress from sitting up and being moved between the bed and a chair to standing, bearing their own weight, and walking, with or without assistance. Rehabilitation nurses and therapists help patients who are able to perform progressively more complex and demanding tasks, such as bathing, dressing, and using a toilet, and they encourage patients to begin using their stroke-impaired limbs while engaging in those tasks. Beginning to reacquire the ability to carry out these basic activities of daily living represents the first stage in a stroke survivor’s return to independence.
For some stroke survivors, rehabilitation will be an ongoing process to maintain and refine skills and could involve working with specialists for months or years after the stroke.
top
What disabilities can result from a stroke?
The types and degrees of disability that follow a stroke depend upon which area of the brain is damaged. Generally, stroke can cause five types of disabilities: paralysis or problems controlling movement; sensory disturbances including pain; problems using or understanding language; problems with thinking and memory; and emotional disturbances.
Paralysis or problems controlling movement (motor control)
Paralysis is one of the most common disabilities resulting from stroke. The paralysis is usually on the side of the body opposite the side of the brain damaged by stroke, and may affect the face, an arm, a leg, or the entire side of the body. This one-sided paralysis is called hemiplegia (one-sided weakness is called hemiparesis). Stroke patients with hemiparesis or hemiplegia may have difficulty with everyday activities such as walking or grasping objects. Some stroke patients have problems with swallowing, called dysphagia, due to damage to the part of the brain that controls the muscles for swallowing. Damage to a lower part of the brain, the cerebellum, can affect the body’s ability to coordinate movement, a disability called ataxia, leading to problems with body posture, walking, and balance.
Sensory disturbances including pain
Stroke patients may lose the ability to feel touch, pain, temperature, or position. Sensory deficits also may hinder the ability to recognize objects that patients are holding and can even be severe enough to cause loss of recognition of one’s own limb. Some stroke patients experience pain, numbness or odd sensations of tingling or prickling in paralyzed or weakened limbs, a symptom known as paresthesias.
The loss of urinary continence is fairly common immediately after a stroke and often results from a combination of sensory and motor deficits. Stroke survivors may lose the ability to sense the need to urinate or the ability to control bladder muscles. Some may lack enough mobility to reach a toilet in time. Loss of bowel control or constipation also may occur. Permanent incontinence after a stroke is uncommon, but even a temporary loss of bowel or bladder control can be emotionally difficult for stroke survivors.
Stroke survivors frequently have a variety of chronic pain syndromes resulting from stroke-induced damage to the nervous system (neuropathic pain). In some stroke patients, pathways for sensation in the brain are damaged, causing the transmission of false signals that result in the sensation of pain in a limb or side of the body that has the sensory deficit. The most common of these pain syndromes is called “thalamic pain syndrome” (caused by a stroke to the thalamus, which processes sensory information from the body to the brain), which can be difficult to treat even with medications. Finally, some pain that occurs after stroke is not due to nervous system damage, but rather to mechanical problems caused by the weakness from the stroke. Patients who have a seriously weakened or paralyzed arm commonly experience moderate to severe pain that radiates outward from the shoulder. Most often, the pain results from lack of movement in a joint that has been immobilized for a prolonged period of time (such as having your arm or shoulder in a cast for weeks) and the tendons and ligaments around the joint become fixed in one position. This is commonly called a “frozen” joint; “passive” movement (the joint is gently moved or flexed by a therapist or caregiver rather than by the individual) at the joint in a paralyzed limb is essential to prevent painful “freezing” and to allow easy movement if and when voluntary motor strength returns.
Problems using or understanding language (aphasia)
At least one-fourth of all stroke survivors experience language impairments, involving the ability to speak, write, and understand spoken and written language. A stroke-induced injury to any of the brain’s language-control centers can severely impair verbal communication. The dominant centers for language are in the left side of the brain for right-handed individuals and many left-handers as well. Damage to a language center located on the dominant side of the brain, known as Broca’s area, causes expressive aphasia. People with this type of aphasia have difficulty conveying their thoughts through words or writing. They lose the ability to speak the words they are thinking and to put words together in coherent, grammatically correct sentences. In contrast, damage to a language center located in a rear portion of the brain, called Wernicke’s area, results in receptive aphasia. People with this condition have difficulty understanding spoken or written language and often have incoherent speech. Although they can form grammatically correct sentences, their utterances are often devoid of meaning. The most severe form of aphasia, global aphasia, is caused by extensive damage to several areas of the brain involved in language function. People with global aphasia lose nearly all their linguistic abilities; they cannot understand language or use it to convey thought.
Problems with thinking and memory
Stroke can cause damage to parts of the brain responsible for memory, learning, and awareness. Stroke survivors may have dramatically shortened attention spans or may experience deficits in short-term memory. Individuals also may lose their ability to make plans, comprehend meaning, learn new tasks, or engage in other complex mental activities. Two fairly common deficits resulting from stroke are anosognosia, an inability to acknowledge the reality of the physical impairments resulting from stroke, and neglect, the loss of the ability to respond to objects or sensory stimuli located on the stroke-impaired side. Stroke survivors who develop apraxia (loss of ability to carry out a learned purposeful movement) cannot plan the steps involved in a complex task and act on them in the proper sequence. Stroke survivors with apraxia also may have problems following a set of instructions. Apraxia appears to be caused by a disruption of the subtle connections that exist between thought and action.
Emotional disturbances
Many people who survive a stroke feel fear, anxiety, frustration, anger, sadness, and a sense of grief for their physical and mental losses. These feelings are a natural response to the psychological trauma of stroke. Some emotional disturbances and personality changes are caused by the physical effects of brain damage. Clinical depression, which is a sense of hopelessness that disrupts an individual’s ability to function, appears to be the emotional disorder most commonly experienced by stroke survivors. Signs of clinical depression include sleep disturbances, a radical change in eating patterns that may lead to sudden weight loss or gain, lethargy, social withdrawal, irritability, fatigue, self-loathing, and suicidal thoughts. Post-stroke depression can be treated with antidepressant medications and psychological counseling.
top
What medical professionals specialize in post-stroke rehabilitation?
Post-stroke rehabilitation involves physicians; rehabilitation nurses; physical, occupational, recreational, speech-language, and vocational therapists; and mental health professionals.
Physicians
Physicians have the primary responsibility for managing and coordinating the long-term care of stroke survivors, including recommending which rehabilitation programs will best address individual needs. Physicians also are responsible for caring for the stroke survivor’s general health and providing guidance aimed at preventing a second stroke, such as controlling high blood pressure or diabetes and eliminating risk factors such as cigarette smoking, excessive weight, a high-cholesterol diet, and high alcohol consumption.
Neurologists usually lead acute-care stroke teams and direct patient care during hospitalization. They sometimes participate on the long-term rehabilitation team. Other subspecialists often lead the rehabilitation stage of care, especially physiatrists, who specialize in physical medicine and rehabilitation.
Rehabilitation nurses
Nurses specializing in rehabilitation help survivors relearn how to carry out the basic activities of daily living. They also educate survivors about routine health care, such as how to follow a medication schedule, how to care for the skin, how to move out of a bed and into a wheelchair, and special needs for people with diabetes. Rehabilitation nurses also work with survivors to reduce risk factors that may lead to a second stroke, and provide training for caregivers.
Nurses are closely involved in helping stroke survivors manage personal care issues, such as bathing and controlling incontinence. Most stroke survivors regain their ability to maintain continence, often with the help of strategies learned during rehabilitation. These strategies include strengthening pelvic muscles through special exercises and following a timed voiding schedule. If problems with incontinence continue, nurses can help caregivers learn to insert and manage catheters and to take special hygienic measures to prevent other incontinence-related health problems from developing.
Physical therapists
Physical therapists specialize in treating disabilities related to motor and sensory impairments. They are trained in all aspects of anatomy and physiology related to normal function, with an emphasis on movement. They assess the stroke survivor’s strength, endurance, range of motion, gait abnormalities, and sensory deficits to design individualized rehabilitation programs aimed at regaining control over motor functions.
Physical therapists help survivors regain the use of stroke-impaired limbs, teach compensatory strategies to reduce the effect of remaining deficits, and establish ongoing exercise programs to help people retain their newly learned skills. Disabled people tend to avoid using impaired limbs, a behavior called learned non-use. However, the repetitive use of impaired limbs encourages brain plasticity and helps reduce disabilities.
Strategies used by physical therapists to encourage the use of impaired limbs include selective sensory stimulation such as tapping or stroking, active and passive range-of-motion exercises, and temporary restraint of healthy limbs while practicing motor tasks.
In general, physical therapy emphasizes practicing isolated movements, repeatedly changing from one kind of movement to another, and rehearsing complex movements that require a great deal of coordination and balance, such as walking up or down stairs or moving safely between obstacles. People too weak to bear their own weight can still practice repetitive movements during hydrotherapy (in which water provides sensory stimulation as well as weight support) or while being partially supported by a harness. A recent trend in physical therapy emphasizes the effectiveness of engaging in goal-directed activities, such as playing games, to promote coordination. Physical therapists frequently employ selective sensory stimulation to encourage use of impaired limbs and to help survivors with neglect regain awareness of stimuli on the neglected side of the body.
Occupational and recreational therapists
Like physical therapists, occupational therapists are concerned with improving motor and sensory abilities, and ensuring patient safety in the post-stroke period. They help survivors relearn skills needed for performing self-directed activities (also called occupations) such as personal grooming, preparing meals, and housecleaning. Therapists can teach some survivors how to adapt to driving and provide on-road training. They often teach people to divide a complex activity into its component parts, practice each part, and then perform the whole sequence of actions. This strategy can improve coordination and may help people with apraxia relearn how to carry out planned actions.
Occupational therapists also teach people how to develop compensatory strategies and change elements of their environment that limit activities of daily living. For example, people with the use of only one hand can substitute hook and loop fasteners (such as Velcro) for buttons on clothing. Occupational therapists also help people make changes in their homes to increase safety, remove barriers, and facilitate physical functioning, such as installing grab bars in bathrooms.
Recreational therapists help people with a variety of disabilities to develop and use their leisure time to enhance their health, independence, and quality of life.
Speech-language pathologists
Speech-language pathologists help stroke survivors with aphasia relearn how to use language or develop alternative means of communication. They also help people improve their ability to swallow, and they work with patients to develop problem-solving and social skills needed to cope with the after-effects of a stroke.
Many specialized therapeutic techniques have been developed to assist people with aphasia. Some forms of short-term therapy can improve comprehension rapidly. Intensive exercises such as repeating the therapist’s words, practicing following directions, and doing reading or writing exercises form the cornerstone of language rehabilitation. Conversational coaching and rehearsal, as well as the development of prompts or cues to help people remember specific words, are sometimes beneficial. Speech-language pathologists also help stroke survivors develop strategies for circumventing language disabilities. These strategies can include the use of symbol boards or sign language. Recent advances in computer technology have spurred the development of new types of equipment to enhance communication.
Speech-language pathologists use special types of imaging techniques to study swallowing patterns of stroke survivors and identify the exact source of their impairment. Difficulties with swallowing have many possible causes, including a delayed swallowing reflex, an inability to manipulate food with the tongue, or an inability to detect food remaining lodged in the cheeks after swallowing. When the cause has been pinpointed, speech-language pathologists work with the individual to devise strategies to overcome or minimize the deficit. Sometimes, simply changing body position and improving posture during eating can bring about improvement. The texture of foods can be modified to make swallowing easier; for example, thin liquids, which often cause choking, can be thickened. Changing eating habits by taking small bites and chewing slowly can also help alleviate dysphagia.
Vocational therapists
Approximately one-fourth of all strokes occur in people between the ages of 45 and 65. For most people in this age group, returning to work is a major concern. Vocational therapists perform many of the same functions that ordinary career counselors do. They can help people with residual disabilities identify vocational strengths and develop résumés that highlight those strengths. They also can help identify potential employers, assist in specific job searches, and provide referrals to stroke vocational rehabilitation agencies.
Most important, vocational therapists educate disabled individuals about their rights and protections as defined by the Americans with Disabilities Act of 1990. This law requires employers to make “reasonable accommodations” for disabled employees. Vocational therapists frequently act as mediators between employers and employees to negotiate the provision of reasonable accommodations in the workplace.
top
When can a stroke patient begin rehabilitation?
Rehabilitation should begin as soon as a stroke patient is stable, sometimes within 24 to 48 hours after a stroke. This first stage of rehabilitation can occur within an acute-care hospital; however, it is very dependent on the unique circumstances of the individual patient.
Recently, in the largest stroke rehabilitation study in the United States, researchers compared two common techniques to help stroke patients improve their walking. Both methods—training on a body-weight supported treadmill or working on strength and balance exercises at home with a physical therapist—resulted in equal improvements in the individual’s ability to walk by the end of one year. Researchers found that functional improvements could be seen as late as one year after the stroke, which goes against the conventional wisdom that most recovery is complete by 6 months. The trial showed that 52 percent of the participants made significant improvements in walking, everyday function and quality of life, regardless of how severe their impairment was, or whether they started the training at 2 or 6 months after the stroke.
top
Where can a stroke patient get rehabilitation?
At the time of discharge from the hospital, the stroke patient and family coordinate with hospital social workers to locate a suitable living arrangement. Many stroke survivors return home, but some move into some type of medical facility.
Inpatient rehabilitation units
Inpatient facilities may be freestanding or part of larger hospital complexes. Patients stay in the facility, usually for 2 to 3 weeks, and engage in a coordinated, intensive program of rehabilitation. Such programs often involve at least 3 hours of active therapy a day, 5 or 6 days a week. Inpatient facilities offer a comprehensive range of medical services, including full-time physician supervision and access to the full range of therapists specializing in post-stroke rehabilitation.
Outpatient units
Outpatient facilities are often part of a larger hospital complex and provide access to physicians and the full range of therapists specializing in stroke rehabilitation. Patients typically spend several hours, often 3 days each week, at the facility taking part in coordinated therapy sessions and return home at night. Comprehensive outpatient facilities frequently offer treatment programs as intense as those of inpatient facilities, but they also can offer less demanding regimens, depending on the patient’s physical capacity.
Nursing facilities
Rehabilitative services available at nursing facilities are more variable than are those at inpatient and outpatient units. Skilled nursing facilities usually place a greater emphasis on rehabilitation, whereas traditional nursing homes emphasize residential care. In addition, fewer hours of therapy are offered compared to outpatient and inpatient rehabilitation units.
Home-based rehabilitation programs
Home rehabilitation allows for great flexibility so that patients can tailor their program of rehabilitation and follow individual schedules. Stroke survivors may participate in an intensive level of therapy several hours per week or follow a less demanding regimen. These arrangements are often best suited for people who require treatment by only one type of rehabilitation therapist. Patients dependent on Medicare coverage for their rehabilitation must meet Medicare’s “homebound” requirements to qualify for such services; at this time lack of transportation is not a valid reason for home therapy. The major disadvantage of home-based rehabilitation programs is the lack of specialized equipment. However, undergoing treatment at home gives people the advantage of practicing skills and developing compensatory strategies in the context of their own living environment. In the recent stroke rehabilitation trial, intensive balance and strength rehabilitation in the home was equivalent to treadmill training at a rehabilitation facility in improving walking.
top
What research is being done?
The National Institute of Neurological Disorders and Stroke (NINDS), a component of the U.S. National Institutes of Health (NIH), has primary responsibility for sponsoring research on disorders of the brain and nervous system, including the acute phase of stroke and the restoration of function after stroke. The NIH’s Eunice Kennedy Shriver National Institute of Child Health and Human Development, through its National Center for Medical Rehabilitation Research, funds work on mechanisms of restoration and repair after stroke, as well as development of new approaches to rehabilitation and evaluation of outcomes. Most of the NIH-funded work on diagnosis and treatment of dysphagia is through the National Institute on Deafness and Other Communication Disorders. The National Institute of Biomedical Imaging and Bioengineering collaborates with NINDS and NICHD in developing new instrumentation for stroke treatment and rehabilitation. The National Eye Institute funds work directed at restoration of vision and rehabilitation for individuals with impaired or low vision that may be due to vascular disease or stroke.
The NINDS supports research on ways to enhance repair and regeneration of the central nervous system. Scientists funded by the NINDS are studying how the brain responds to experience or adapts to injury by reorganizing its functions (plasticity)—using noninvasive imaging technologies to map patterns of biological activity inside the brain. Other NINDS-sponsored scientists are looking at brain reorganization after stroke and determining whether specific rehabilitative techniques, such as constraint-induced movement therapy and transcranial magnetic stimulation, can stimulate brain plasticity, thereby improving motor function and decreasing disability. Other scientists are experimenting with implantation of neural stem cells, to see if these cells may be able to replace the cells that died as a result of a stroke.
*An ischemic stroke or “brain attack” occurs when brain cells die because of inadequate blood flow. When blood flow is interrupted, brain cells are robbed of vital supplies of oxygen and nutrients. About 80 percent of strokes are caused by the blockage of an artery in the neck or brain. A hemorrhagic stroke is caused by a burst blood vessel in the brain that causes bleeding into or around the brain.
**Functions compromised when a specific region of the brain is damaged by stroke can sometimes be taken over by other parts of the brain. This ability to adapt and change is known as neuroplasticity.
top
Where can I get more information?
For more information on neurological disorders or research programs funded by the National Institute of Neurological Disorders and Stroke, contact the Institute’s Brain Resources and Information Network (BRAIN) at:
BRAIN
P.O. Box 5801
Bethesda, MD 20824
800-352-9424
http://www.ninds.nih.gov
Information also is available from the following organizations:
American Stroke Association: A Division of American Heart Association
7272 Greenville Avenue
Dallas, TX 75231-4596
Tel: 888-4STROKE (478-7653)
Brain Aneurysm Foundation
269 Hanover Street, Building 3
Hanover, MA 02339
Tel: 781-826-5556; 888-BRAIN02 (272-4602)
Brain Attack Coalition
31 Center Drive
Room 8A07
Bethesda, MD 20892-2540
Tel: 301-496-5751
Children’s Hemiplegia and Stroke Assocn. (CHASA)
4101 West Green Oaks Blvd., Ste. 305
PMB 149
Arlington, TX 76016
Tel: 817-492-4325
Fibromuscular Dysplasia Society of America (FMDSA)
20325 Center Ridge Road Suite 620
Rocky River, OH 44116
Tel: 216-834-2410; 888-709-7089
Hazel K. Goddess Fund for Stroke Research in Women
785 Park Road, #3E
New York, NY 10021
Heart Rhythm Society
1325 G Street, N.W.
Suite 400
Washington, DC 20005
Tel: 202-464-3454
Joe Niekro Foundation
PO Box 2876
Scottsdale, AZ 85252
Tel: 602-318-1013
National Aphasia Association
P.O. Box 87
Scarsdale, NY 10583
Tel: 212-267-2814; 800-922-4NAA (4622)
National Stroke Association
9707 East Easter Lane
Suite B
Centennial, CO 80112-3747
Tel: 303-649-9299; 800-STROKES (787-6537)
YoungStroke, Inc.
P.O. Box 692
Conway, SC 29528
Tel: 843-248-9019; 843-655-2835
“Post-Stroke Fact Sheet”, NINDS, Publication date September 2014.
NIH Publication No. 14-1846
Stroke fact sheet available in multiple languages through MedlinePlus
Back to Stroke Information
See a list of all NINDS disorders
Publicaciones en Español
Ataque Cerebral
Prepared by:
Office of Communications and Public Liaison
National Institute of Neurological Disorders and Stroke
National Institutes of Health
Bethesda, MD 20892
NINDS health-related material is provided for information purposes only and does not necessarily represent endorsement by or an official position of the National Institute of Neurological Disorders and Stroke or any other Federal agency. Advice on the treatment or care of an individual patient should be obtained through consultation with a physician who has examined that patient or is familiar with that patient’s medical history.
All NINDS-prepared information is in the public domain and may be freely copied. Credit to the NINDS or the NIH is appreciated.





















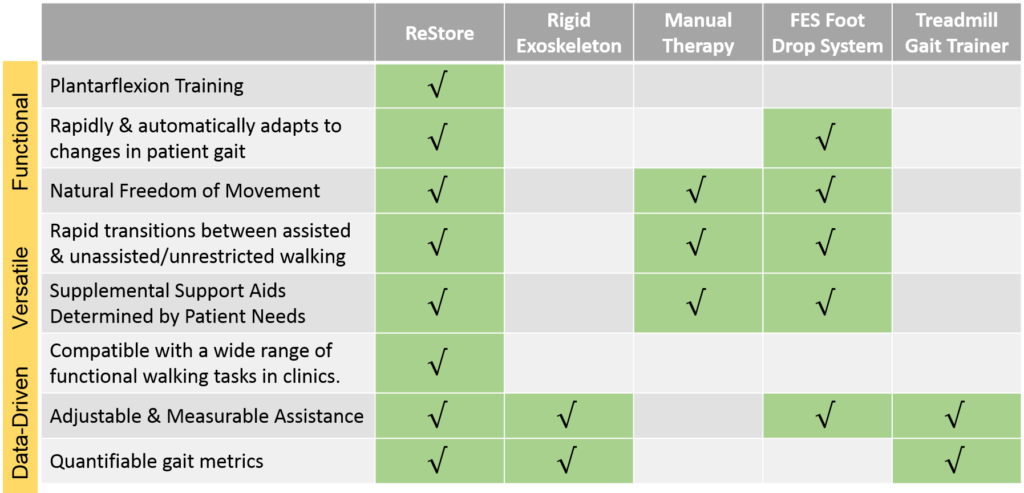
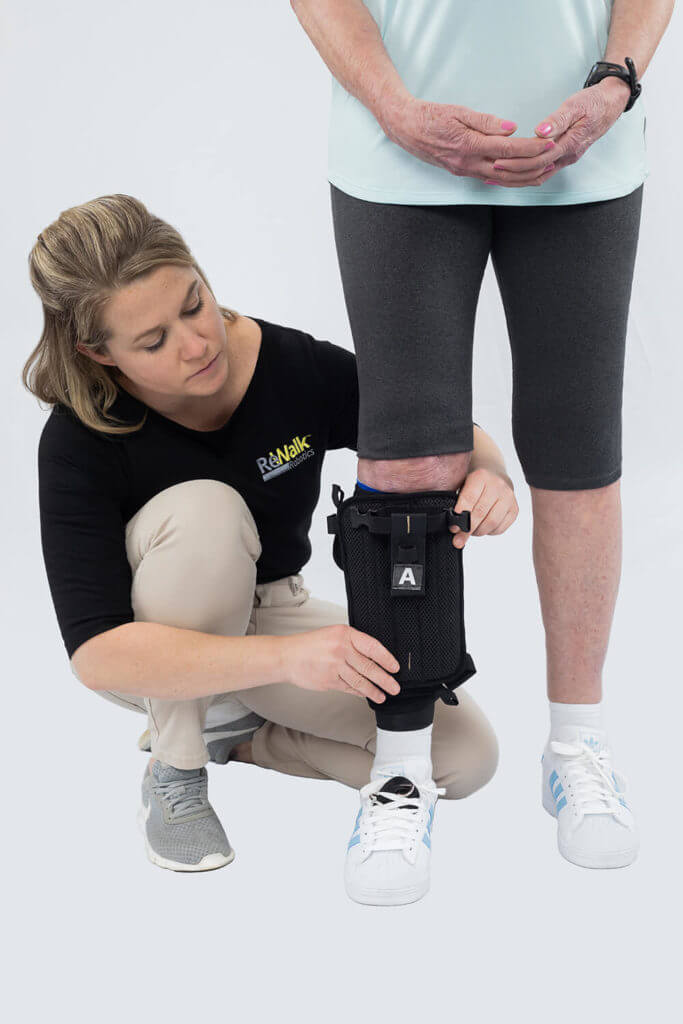 The ReStore is a powered, lightweight soft exo-suit intended for use in the rehabilitation of persons with lower limb disability due to stroke. It will be a first of its kind gait training solution.
The ReStore is a powered, lightweight soft exo-suit intended for use in the rehabilitation of persons with lower limb disability due to stroke. It will be a first of its kind gait training solution.