Posts Tagged locomotion
[BLOG POST] Smartphone app quickly analyzes human motion to aid physical rehab
Posted by Kostas Pantremenos in Apps, Gait Rehabilitation - Foot Drop, REHABILITATION on October 20, 2023
A research team funded by the National Institutes of Health has developed a smart phone app that can track and analyze a person’s ability to move from one place to another, known as locomotion, and other types of movements. Human motion analysis is used to evaluate patients with movement difficulties, to help clinicians plan surgery, and to assess the results of treatment procedures. The research team believes that using the app costs about 1% of conventional motion analysis techniques and works 25 times faster. The study appears in PLOS Computational Biology.
[ARTICLE] Feasibility and Usability of a Robot-Assisted Complex Upper and Lower Limb Rehabilitation System in Patients with Stroke: A Pilot Study – Full Text
Posted by Kostas Pantremenos in Gait Rehabilitation - Foot Drop, Paretic Hand, Rehabilitation robotics on May 4, 2023
Abstract
Objective
To evaluate the feasibility and usability of cost-effective complex upper and lower limb robot-assisted gait training in patients with stroke using the GTR-A, a foot-plate based end-effector type robotic device.
Methods
Patients with subacute stroke (n=9) were included in this study. The enrolled patients received 30-minute robot-assisted gait training thrice a week for 2 weeks (6 sessions). The hand grip strength, functional ambulation categories, modified Barthel index, muscle strength test sum score, Berg Balance Scale, Timed Up and Go Test, and Short Physical Performance Battery were used as functional assessments. The heart rate was measured to evaluate cardiorespiratory fitness. A structured questionnaire was used to evaluate the usability of robot-assisted gait training. All the parameters were evaluated before and after the robot-assisted gait training program.
Results
Eight patients completed robot-assisted gait training, and all parameters of functional assessment significantly improved between baseline and posttraining, except for hand grip strength and muscle strength test score. The mean scores for each domain of the questionnaire were as follows: safety, 4.40±0.35; effects, 4.23±0.31; efficiency, 4.22±0.77; and satisfaction, 4.41±0.25.
Conclusion
Thus, the GTR-A is a feasible and safe robotic device for patients with gait impairment after stroke, resulting in improvement of ambulatory function and performance of activities of daily living with endurance training. Further research including various diseases and larger sample groups is necessary to verify the utility of this device.
GRAPHICAL ABSTRACT

Go to : 
INTRODUCTION
Restoration of walking ability is one of the primary therapeutic goals of stroke rehabilitation [1,2]. Gait disturbance in patients with hemiparetic stroke reduces social participation and quality of life and increases socioeconomic burden and mortality [3]. Robot-assisted gait training (RAGT) has been highlighted as an efficient intervention after stroke that provides task-specific training similar to actual gait in the early stages of recovery [4,5]. RAGT can provide abundant repetitive tasks that facilitate the integration of the remaining sensory and motor functions and help reorganize the motor engram [6]. Bilateral, reciprocal upper and lower limb locomotor training enhances cortical reorganization [7], and selfpaced treadmill walking simulating actual gait improves brain activity with higher cognitive engagement in stroke survivors [8]. However, commercially available gait training robotic devices mainly focus on the recovery of lower extremity function [9,10]. Even if a handrail is used for balance and body weight support, it cannot provide reciprocal movements of the upper and lower limbs during gait training.
Although recovery of balance, motor strength, and control are crucial for gait function in patients with stroke, facilitating cardiorespiratory fitness (CRF) is also an important goal in gait rehabilitation. Gait impairment can reduce physical tolerance, which leads to a sedentary lifestyle and can result in further sarcopenia and osteoporosis [6,11]. These complications generate a vicious cycle in which patients’ decreased cardiorespiratory endurance further limits their physical activity. Recently, robotic devices have been considered as an alternative tool for endurance training in physically disabled patients [12]. However, most gait training robotic devices only provide entirely passive gait training, regardless of the voluntary engagement of the patient, and exercise intensity is much lower than that of independent self-gait. The G-EO (Reha Technology AG, Olten, Switzerland) and RT600 (Restorative Therapies, Nottingham, MD, USA) have a partial assist mode and hybrid rehabilitation systems that can provide additional functional electrical stimulation along with the gait cycle. However, these methods are expensive, and their use is limited [13,14].
The purpose of this study was to develop a robotassisted complex upper and lower limb rehabilitation system that can implement reciprocal movements similar to actual gait. We aimed to evaluate the feasibility and usability of the newly developed GTR-A (HUCASYSTEM, Sejong, Korea) robotic device. We hypothesized that gait training using the GTR-A is safe and has an endurance training effect with functional gain.[…]
[Abstract] Feasibility and Usability Study of a Robot-Assisted Complex Upper and Lower Limb Rehabilitation System in Patients with Stroke[v1]
Posted by Kostas Pantremenos in Gait Rehabilitation - Foot Drop, Paretic Hand, Rehabilitation robotics on August 14, 2022
Abstract
Robot-assisted gait training (RAGT) is a promising treatment for stroke rehabilitation. Although the coordination between the upper and lower limbs is important for locomotor training, commercially available robotics for gait training mainly focus on the restoration of lower limb function. We aimed to evaluate the feasibility and usability of complex upper and lower limb RAGT in stroke patients using the GTR-A®, end effector-type robotic device. Patients with subacute stroke (N=9) received 30-minute RAGT thrice a week for two weeks (six sessions). Functionally, the hand grip strength (HGS), Functional Ambulatory Categories, modified Barthel Index, muscle strength test sum score, Berg Balance Scale, Timed Up and Go test, and Short Physical Performance Battery were used. The heart rate and a structured questionnaire were used to evaluate cardiorespiratory fitness and the usability of RAGT. Among the nine patients, all functional parameters between the baseline and post-training were significantly improved after RAGT, except for HGS and the muscle strength test. The questionnaire’s mean scores for each domain were as follows: safety 4.40±0.35, effects 4.23±0.31, efficiency 4.22±0.77, and satisfaction 4.41±0.25. The GTR-A® is a feasible and safe robotic device for patients with gait impairment after stroke. It showed functional improvement with endurance training effects.
[Abstract] Gains in Daily Stepping Activity in People with Chronic Stroke after High-Intensity Gait Training in Variable Contexts
Posted by Kostas Pantremenos in Gait Rehabilitation - Foot Drop on July 17, 2022
Abstract
Objective. Many physical therapist interventions provided to individuals with chronic stroke can lead to gains in gait speed or endurance (eg, 6-Minute Walk Test [6MWT]), although changes in objective measures of participation are not often observed. The goal of this study was to determine the influence of different walking interventions on daily stepping (steps per day) and the contributions of demographic, training, and clinical measures to these changes.
Methods. In this secondary analysis of a randomized clinical trial, steps per day at baseline and changes in steps per day following 1 of 3 locomotor interventions were evaluated in individuals who were ambulatory and > 6 months after stroke. Data were collected on 58 individuals who received ≤30 sessions of high-intensity training (HIT) in variable contexts (eg, tasks and environments; n = 19), HIT focused on forward walking (n = 19), or low-intensity variable training (n = 20). Primary outcomes were steps per day at baseline, after training, and at a 3-month follow-up, and secondary outcomes were gait speed, 6MWT, balance, and balance confidence. Correlation and regression analyses identified demographic and clinical variables associated with steps per day.
Results. Gains in steps per day were observed across all groups combined, with no between-group differences; post hoc within-group analyses revealed significant gains only following HIT in variable contexts. Both HIT groups showed gains in endurance (6MWT), with increases in balance confidence only following HIT in variable contexts. Changes in steps per day were associated primarily with gains in 6MWT, with additional associations with baseline 6MWT, lower-extremity Fugl-Meyer scores, and changes in balance confidence.
Conclusion. HIT in variable contexts elicited gains in daily stepping, with changes primarily associated with gains in gait endurance.
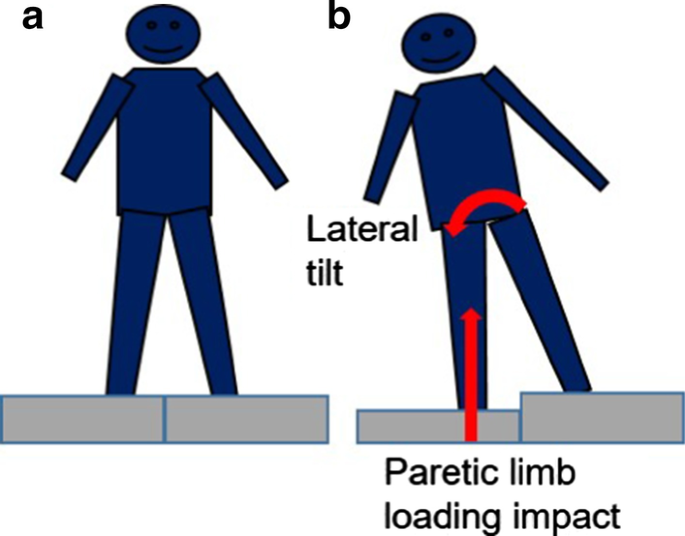
[ARTICLE] Biomechanical control of paretic lower limb during imposed weight transfer in individuals post-stroke – Full Text
Posted by Kostas Pantremenos in Gait Rehabilitation - Foot Drop, REHABILITATION on November 24, 2020
Abstract
Background
Stroke is a leading cause of disability with associated hemiparesis resulting in difficulty bearing and transferring weight on to the paretic limb. Difficulties in weight bearing and weight transfer may result in impaired mobility and balance, increased fall risk, and decreased community engagement. Despite considerable efforts aimed at improving weight transfer after stroke, impairments in its neuromotor and biomechanical control remain poorly understood. In the present study, a novel experimental paradigm was used to characterize differences in weight transfer biomechanics in individuals with chronic stroke versus able-bodied controls
Methods
Fifteen participants with stroke and fifteen age-matched able-bodied controls participated in the study. Participants stood with one foot on each of two custom built platforms. One of the platforms dropped 4.3 cm vertically to induce lateral weight transfer and weight bearing. Trials involving a drop of the platform beneath the paretic lower extremity (non-dominant limb for control) were included in the analyses. Paretic lower extremity joint kinematics, vertical ground reaction forces, and center of pressure velocity were measured. All participants completed the clinical Step Test and Four-Square Step Test.
Results
Reduced paretic ankle, knee, and hip joint angular displacement and velocity, delayed ankle and knee inter-joint timing, increased downward displacement of center of mass, and increased center of pressure (COP) velocity stabilization time were exhibited in the stroke group compared to the control group. In addition, paretic COP velocity stabilization time during induced weight transfer predicted Four-Square Step Test scores in individuals post-stroke.
Conclusions
The induced weight transfer approach identified stroke-related abnormalities in the control of weight transfer towards the paretic limb side compared to controls. Decreased joint flexion of the paretic ankle and knee, altered inter-joint timing, and increased COP stabilization times may reflect difficulties in neuromuscular control during weight transfer following stroke. Future work will investigate the potential of improving functional weight transfer through induced weight transfer training exercise.
Background
Stroke is a leading cause of death and serious long-term disability in the United States [1, 2]. Individuals with hemiparesis due to stroke commonly demonstrate difficulty bearing weight on the paretic lower extremity and transferring weight from one leg to the other [3,4,5]. Reduced paretic limb weight bearing has been associated with functional deficits when rising from a chair [6], standing [7], and walking [8, 9]. The ability to transfer bodyweight between the lower limbs is related to impaired standing and stepping balance [10, 11] and gait performance [3, 12]. In particular, diminished weight transfer to the paretic limb contributes to gait asymmetries, which commonly lead to greater energy expenditure [13]. Previously we reported the ability to transfer weight laterally to the paretic leg during single stance was associated with self-selected walking speed and the capacity to increase walking speed [14]. This may indicate that weight transfer deficits negatively affect forward progression. Indeed, forceful weight shift towards the paretic limb enhanced paretic lower extremity kinetics and muscle activities that contribute to forward progression [15]. Moreover, deficits in paretic limb weight-bearing contribute to lateral and vertical balance instability and are associated with risk of falling in individuals with chronic stroke [16]. These functional limitations can affect community participation and quality of life. Consequently, restoring the capacity to load the paretic limb is an important goal for rehabilitation post-stroke [17,18,19].
Despite considerable rehabilitation efforts aimed at improving weight transfer following a stroke [10, 20], the impairments in neuromotor and biomechanical control underlying weight transfer dysfunction remain poorly understood. Functional weight transfer requires the coordination of multi-joint actions to absorb the impact force and provide support to the body. In particular, the ankle and knee joints are key contributors to shock absorption [21,22,23,24] and body weight support [25]. Increased stiffness in the paretic limb knee and ankle joints has been reported in persons with stroke [26, 27]. Inadequate lower limb joint flexion may disrupt impact force regulation during weight acceptance and lead to instability that ultimately delays and prolongs weight transfer timing during locomotion. Alternatively, excessive ankle and knee joint flexion during loading may precipitate limb collapse and destabilize balance during weight transfer. Thus, both insufficient and excessive joint movement could affect weight transfer processes. In addition to the amplitude of paretic ankle and knee joint angular displacements, abnormalities in the relative timing of these joint motions (i.e., inter-joint coordination) may also contribute to impaired weight transfer following stroke.
Another key factor affecting functional weight transfer is the ability to regulate the center of pressure (COP) beneath the feet in relation to the body center of mass (COM). During locomotion, effective neuromotor control of the lower extremities contributes to regulating COM position and movement relative to the base of support to maintain stability and prevent falling. Compared with able-bodied adults, persons with chronic stroke have a reduced capacity to rapidly shift their COP to the stance limb during gait initiation [28], reflecting abnormalities in balance control during weight transfer. Because hip and ankle musculature regulates COM and COP movements [29], difficulties in controlling hip kinematics and hip-ankle joint coordination may contribute to delayed and reduced weight transfer after a stroke.
To further address the foregoing issues, this study examined the potential biomechanical factors that could affect lower paretic limb weight bearing and weight transfer performance following stroke. After stroke individuals often limit their use of the paretic limb by favoring the use of the less affected leg during stance and gait [30]. An approach that forces individuals to fully load the paretic limb is warranted to reveal the performance capacity and assess the control of weight bearing and weight transfer. Accordingly, we designed a novel system that vertically displaces the support surface underneath one leg and therefore imposes weight transfer. By unilaterally introducing a perturbation that drops the standing support surface, this approach forces a rapid alteration in inter-limb weight bearing distribution and challenges medial–lateral balance control.
The primary purpose of this study was to characterize the kinematics and kinetics of the paretic lower extremity during an externally induced weight transfer towards the paretic limb in chronic stroke compared to age-matched controls. We hypothesized that, compared with able-bodied individuals, those with chronic stroke would show reduced and uncoordinated paretic limb joint angular displacements, and prolonged stabilization time of the COP velocity following an externally induced weight transfer. In addition, relationships between measurements during imposed weight transfer, motor recovery assessment (i.e. Chedoke McMaster Stroke Assessment leg and foot subscale), and clinical limb loading and balance performance (i.e. Four-Square Step Test (FSST) and Step Test (ST)) were explored. We expected that COP velocity stabilization time and CMSA scores would be associated with FSST and ST scores.[…]
[ARTICLE] Contributions of Stepping Intensity and Variability to Mobility in Individuals Poststroke – Full Text
Posted by Kostas Pantremenos in Gait Rehabilitation - Foot Drop on August 22, 2020
Abstract
Background and Purpose—
The amount of task-specific stepping practice provided during rehabilitation poststroke can influence locomotor recovery and reflects one aspect of exercise dose that can affect the efficacy of specific interventions. Emerging data suggest that markedly increasing the intensity and variability of stepping practice may also be critical, although such strategies are discouraged during traditional rehabilitation. The goal of this study was to determine the individual and combined contributions of intensity and variability of stepping practice to improving walking speed and distance in individuals poststroke.
Methods—
This phase 2, randomized, blinded assessor clinical trial was performed between May 2015 and November 2018. Individuals between 18 and 85 years old with hemiparesis poststroke of >6 months duration were recruited. Of the 152 individuals screened, 97 were randomly assigned to 1 of 3 training groups, with 90 completing >10 sessions. Interventions consisted of either high-intensity stepping (70%–80% heart rate reserve) of variable, difficult stepping tasks (high variable), high-intensity stepping performing only forward walking (high forward), and low-intensity stepping in variable contexts at 30% to 40% heart rate reserve (low variable). Participants received up to 30 sessions over 2 months, with testing at baseline, post-training, and a 3-month follow-up. Primary outcomes included walking speeds and timed distance, with secondary measures of dynamic balance, transfers, spatiotemporal kinematics, and metabolic measures.
Results—
All walking gains were significantly greater following either high-intensity group versus low-variable training (all P<0.001) with significant correlations with stepping amount and rate (r=0.48–60; P<0.01). Additional gains in spatiotemporal symmetry were observed with high-intensity training, and balance confidence increased only following high-variable training in individuals with severe impairments.
Conclusions—
High-intensity stepping training resulted in greater improvements in walking ability and gait symmetry than low-intensity training in individuals with chronic stroke, with potential greater improvements in balance confidence.
Introduction
The increasing incidence1 and current survival rates of individuals who experience a stroke have resulted in a substantial patient population with neurological deficits that limit locomotor capacity and postural stability.2,3 In individuals with chronic (>6 months) stroke, mobility limitations4,5 lead to reduced cardiopulmonary capacity that can further exacerbate locomotor deficits.3 Previous work6,7 suggests specific exercise training parameters, including the frequency, intensity, time, and type, can influence changes in health and fitness in individuals with and without neurological injury.8 These parameters represent the dose of exercise interventions, although their contributions to locomotor recovery poststroke are uncertain. Early studies advocated that large amounts of stepping practice with focus on normalizing gait patterns was a critical determinant of improved mobility.9–11 Unfortunately, a multicenter trial using this strategy revealed limited gains beyond conventional approaches.12 Additional research indicates treadmill exercise at submaximal aerobic intensities determined during baseline testing can improve walking endurance poststroke,13–15 although changes in walking speed or other mobility outcomes (balance or transfers) are inconsistent or negligible. The combined findings imply that these dosage parameters may not be critical to locomotor recovery poststroke.
An alternative hypothesis is that specific training variables can influence locomotor recovery when their manipulation substantially challenges the physiological demands associated with functional mobility. In particular, pilot studies indicate stepping training at cardiovascular intensities that are oftentimes greater than those achieved during baseline testing can improve multiple measures of locomotor and cardiopulmonary function.16–18 In addition, increasing the variability and difficulty of stepping tasks (eg, multidirectional walking, stair climbing, overground walking on uneven, or compliant surfaces) requires increased neuromuscular coordination and postural control that may improve mobility and dynamic stability.16,17,19
Despite these findings, clinical implementation of high-intensity stepping training in variable contexts is limited. Specific concerns include the potential for cardiovascular events,20 despite data indicating no additional risks compared to standard interventions.21 Additional concerns include practice of abnormal kinematic strategies, particularly in those with severe neuromuscular impairments during difficult, variable tasks. Such training deviates considerably from traditional interventions that focus on correcting abnormal gait patterns,9,10,12 although available data suggest gait kinematics can improve with variable stepping training.16,17,22
The present study examined the relative contributions of stepping intensity and variability on mobility outcomes in ambulatory individuals with chronic stroke. Using a randomized, controlled trial design, we hypothesized that high-intensity stepping training in variable contexts would result in greater gains in locomotor outcomes as compared to more traditional training focused on forward walking or low-intensity training of variable stepping tasks. Additional outcomes included alterations in transfers, dynamic balance and balance confidence, spatiotemporal kinematics, peak metabolic capacity, and potential adverse events. Results from this trial could indicate the potential utility of high-intensity training of variable, difficult tasks to improve mobility poststroke.[…]
Source: https://www.ahajournals.org/doi/10.1161/STROKEAHA.119.026254
[Abstract] Locomotor Training Intensity After Stroke: Effects of Interval Type and Mode
Posted by Kostas Pantremenos in Gait Rehabilitation - Foot Drop, REHABILITATION on May 5, 2020
Abstract
Background and Objectives: High-intensity interval training (HIIT) is a promising strategy for improving gait and fitness after stroke, but optimal parameters remain unknown. We tested the effects of short vs long interval type and over-ground vs treadmill mode on training intensity.
Methods: Using a repeated measures design, 10 participants with chronic hemiparesis performed 12 HIIT sessions over 4 weeks, alternating between short and long-interval HIIT sessions. Both protocols included 10 minutes of over-ground HIIT, 20 minutes of treadmill HIIT and another 10 minutes over-ground. Short-interval HIIT involved 30 second bursts at maximum safe speed and 30-60 second rest periods. Long-interval HIIT involved 4-minute bursts at ~90% of peak heart rate (HRpeak) and 3-minute recovery periods at ~70% HRpeak.
Results: Compared with long-interval HIIT, short-interval HIIT had significantly faster mean overground speeds (0.75 vs 0.67 m/s) and treadmill speeds (0.90 vs 0.51 m/s), with similar mean treadmill HR (82.9 vs 81.8%HRpeak) and session perceived exertion (16.3 vs 16.3), but lower overground HR (78.4 vs 81.1%HRpeak) and session step counts (1481 vs 1672). For short-interval HIIT, training speeds and HR were significantly higher on the treadmill vs. overground. For long-interval HIIT, the treadmill elicited HR similar to overground training at significantly slower speeds.
Conclusions: Both short and long-interval HIIT elicit high intensities but emphasize different dosing parameters. From these preliminary findings and previous studies, we hypothesize that overground and treadmill short-interval HIIT could be optimal for improving gait speed and overground long-interval HIIT could be optimal for improving gait endurance.
via Locomotor Training Intensity After Stroke: Effects of Interval Type and Mode – PubMed
[ARTICLE] Minimal Clinically Important Difference of the 6-Minute Walk Test in People With Stroke – Full Text
Posted by Kostas Pantremenos in Gait Rehabilitation - Foot Drop on October 31, 2018
Abstract
Background and Purpose: The 6-minute walk test (6MWT) is commonly used in people with stroke. The purpose of this study was to estimate the minimal clinically important difference (MCID) of the 6MWT 2 months poststroke.
Methods: We performed a secondary analysis of data from a rehabilitation trial. Participants underwent physical therapy between 2 and 6 months poststroke and the 6MWT was measured before and after. Two anchors of important change were used: the modified Rankin Scale (mRS) and the Stroke Impact Scale (SIS). The MCID for the 6MWT was estimated using receiver operating characteristic curves for the entire sample and for 2 subgroups: initial gait speed (IGS) <0.40 m/s and ≥0.40 m/s.
Results: For the entire sample, the estimated MCID of the 6MWT was 71 m with the mRS as the anchor (area under the curve [AUC] = 0.66) and 65 m with the SIS as the anchor (AUC = 0.59). For participants with IGS <0.40 m/s, the estimated MCID was 44 m with the mRS as the anchor (AUC = 0.72) and 34 m with the SIS as the anchor (AUC = 0.62). For participants with IGS ≥0.40 m/s, the estimated MCID was 71 m with the mRS as the anchor (AUC = 0.59) and 130 m with the SIS as the anchor (AUC = 0.56).
Discussion and Conclusions: Between 2 and 6 months poststroke, people whose IGS is <0.40 m/s and experience a 44-m improvement in the 6MWT may exhibit meaningful improvement in disability. However, we were not able to estimate an accurate MCID for the 6MWT in people whose IGS was ≥0.40 m/s. MCID values should be estimated across different levels of function and anchors of importance.
Video Abstract available for more insights from the authors (see Video, Supplemental Digital Content 1, available at: http://links.lww.com/JNPT/A232).
INTRODUCTION
The 6-minute walk test (6MWT) is commonly used in people with stroke undergoing rehabilitation.1–3 Although originally developed and validated as a submaximal oxygen consumption test for individuals with cardiac or pulmonary disease,4 , 5 the 6MWT is a valid6–11and reliable12 , 13 measure of walking endurance and is highly recommended by the Academy of Neurologic Physical Therapy for use with people with stroke and other neurologic conditions across the continuum of care.14 More recently, the 6MWT has been used to predict community walking activity.15
An important psychometric property of any outcome measure is its sensitivity to change and responsiveness. Liang and colleagues16 , 17 define sensitivity to change as the ability of an instrument to measure change regardless of whether or not that change is important; it is the amount of change that exceeds measurement error and patient variability. Responsiveness is the ability of an instrument to measure important change. In particular, the minimal detectable change ([MDC] an index of sensitivity to change) and the minimal clinically important difference ([MCID] an index of responsiveness) are useful for clinicians and researchers when interpreting scores and/or change on an outcome measure. The MDC is an estimate of the measurement error and random fluctuation in the test score in patients who are stable.18 , 19
Although MDC is useful for interpreting change scores, it is not ideal, as it provides only the information that the change has exceeded measurement error and variability in patients who are stable. Conversely, the MCID is more useful clinically as it provides an index of important change. The MCID involves an anchor-based approach to estimating how much change in an outcome measure is clinically important and meaningful. The anchor is some external variable that is judged to be important.20 External anchors can be patients’ perception of important change, clinicians’ perception of important change, or an objective marker of important change (eg, discharge home).20 For example, Fulk and colleagues21 used patient and therapist’s perception of important change measured with a Global Rating of Change Scale as an anchor to estimate clinically important change in the Arm Motor Ability Test. When estimating the MCID of gait speed, Tilson and colleagues22 used a 1-point improvement on the modified Rankin Scale (mRS) as the anchor of important improvement in disability.
Unfortunately, there is limited research on the sensitivity to change and responsiveness of the 6MWT in people with stroke. In people with chronic stroke, the MDC is estimated to be 29 m,12 ,23 while in people with stroke undergoing inpatient rehabilitation 30 days poststroke, the MDC is estimated to be 54 m.10 To the best of our knowledge, the MCID of the 6MWT has been reported for people with stroke in only 1 other study. Using data from a completed rehabilitation trial, Perera and colleagues24 estimated meaningful change in the 6MWT using 3 different methodologies. They used an anchor-based approach using decline on 2 items of the 36-Item Short Form Health Survey (walking 1 block and climbing a flight of stairs) as the anchors. Using a distribution-based approach, they calculated standard error of measurement, and they multiplied mean baseline 6MWT distance by a small (0.2) and medium effect size (0.5). Limitations in their findings are that the anchor-based approach used was in relation to decline in performance on the anchor and so should not be applied when trying to interpret improvement. The distribution-based methods Perera and colleagues24 used to estimate change in the 6MWT were based on patients whose condition was stable and are indices of sensitivity to change not responsiveness (ie, important change). However, the MCID of the 6MWT has been reported for other patient populations and has been estimated to be between 14.0 m and 156 m in people with chronic obstructive pulmonary disease, lung disease (lung disease), coronary artery disease, fibromyalgia, and older adults.25–28
The purpose of this research study was to estimate the MCID of the 6MWT in people with stroke undergoing outpatient rehabilitation 2 months poststroke using an anchor-based approach. Based on the MDC values reported in the literature, we hypothesized that the MCID would be greater than the reported MDC values.[…]
Continue —> Minimal Clinically Important Difference of the 6-Minute Walk… : Journal of Neurologic Physical Therapy
[Abstract] Early robot-assisted gait retraining in non-ambulatory patients with stroke: a single blind randomized controlled trial
Posted by Kostas Pantremenos in Gait Rehabilitation - Foot Drop, Rehabilitation robotics on April 11, 2018
BACKGROUND: Restoration of walking function is a primary concern of neurorehabilitation with respect to the aspired social and vocational reintegration. To date, the best practice for improving gait early after stroke is still object of debate. On one hand, repetitive task-specific approaches with higher intensities of walking have been observed to result in greater improvements of gait after stroke. Conversely there is some evidence that conventional gait training would be more effective for facilitating walking ability after stroke.
AIM: To compare the effects of an early treatment protocol of add-on robot-assisted gait training with add-on conventional overground physiotherapy for improving locomotion in non-ambulatory adult stroke patients.
DESIGN: Single-blind randomized controlled trial.
SETTING: Neurorehabilitation hospital.
POPULATION: Seventy-four subacute patients with first-ever ischemic stroke.
METHODS: The patients were randomized into two groups. The training program consisted of forty, 2-hour sessions (including 45 minutes basic training, 45 minutes add-on training plus rest periods), five days a week, for eight consecutive weeks. Patients allocated to the add-on robot-assisted gait training were treated by means of the Lokomat. Patients allocated to the add-on conventional overground gait training aimed at improving postural control during gait, body weight transfer, stability during the stance phase, free swing phase, adequate heel contact and gait pattern. Primary outcome was the modified Emory Functional Ambulation Profile. Secondary outcomes were the Rivermead Motor Index, the Mobility Milestones and the Hochzirl Walking Aids Profile.
RESULTS: No significant difference was observed between groups with regards to age (P=0.661), time from stroke onset (P=0.413) and the primary outcome (P=0.854) at baseline evaluation. As to the primary outcome, no significant differences were found between groups at the end of the study. As During the 8-week training, within-group comparisons showed significant improvements of mean modified Emory Functional Ambulation Profile in both groups (P<0.001).
CONCLUSIONS: Our results support the hypothesis that an early treatment protocol of robot-assisted gait retraining is not superior to add-on conventional gait training intervention for improving locomotion in non- ambulatory stroke patients.
CLINICAL REHABILITATION IMPACT: This study might help to better understand the role of robot-assisted gait training in early phase stroke rehabilitation.
Full Text PDF
[Abstract] Efficacy of the Regent Suit-based rehabilitation on gait EMG patterns in hemiparetic subjects: a pilot study
Posted by Kostas Pantremenos in Gait Rehabilitation - Foot Drop on January 17, 2018
Abstract
Background: The recovery of the functional limb mobility of patients with cerebral damages can take great benefit of the role offered by proprioceptive rehabilitation. Recently have been developed a special Regent Suit (RS) for rehabilitative applications. Actually, there are preliminary studies which describes the effects of RS on gait recovery of stroke patients in acute stage, but none in chronic stage. Moreover, it is known that motor recovery does not always reflect improvements of the muscle activity and coactivity.
AIM: To investigate the effects of proprioceptive stimulation induced by the Regent Suit (RS) on the EMG patterns during gait in post-stroke chronic patients.
Design: Randomized controlled trial.
Setting: S. Maugeri Foundation, Telese Terme (BN), Italy.
Population: Patients have been randomly assigned into two equal groups of 20 patients: experimental group and traditional group. Further, a control group of 20 healthy subjects have been enrolled.
Settings: The traditional group attended a rehabilitation program composed by neuro-motor exercises without the RS, the experimental group performed the same rehabilitation program while wearing the RS. the NIH Stroke Scale (NIHSS), the Barthel Index (BI), the Functional Independent Measure (FIM) and the Berg Balance Scale (BBS) have been evaluated. EMG analysis has been performed considering the muscle activation timing over the gait of the Soleus, Tibialis Anterior, Semitendinosus and Vastus Lateralis muscles by decomposing the EMG signals into Gaussian pulses. Then, the symmetry of muscle activation and the muscle synergy patterns over the gait cycle have been assessed.
Results: The proprioceptive stimulation of the RS-based treatment induces higher and remarkable restoration of the normal muscle activation timing, also increasing the muscle symmetry and reducing the pathological muscle coactivation on both affected and non-affected sides.
Conclusions: These results suggest confirm that a RS-based treatment is more effective than usual care in improving the EMG patterns during locomotion and daily living activities in chronic post-stroke subjects.
Clinical Rehabilitation Impact: The proprioceptive rehabilitation Regent Suit based has an impact on motor function in stroke patients during gait.
Download Full Test PDF
