Posts Tagged botulinum toxin
[ARTICLE] Robotic gait training and botulinum toxin injection improve gait in the chronic post-stroke phase: A randomized controlled trial
Posted by Kostas Pantremenos in Gait Rehabilitation - Foot Drop, Pharmacological, Rehabilitation robotics on December 23, 2023
Abstract
Background
Improving walking ability is one of the main goals of rehabilitation after stroke. When lower limb spasticity increases walking difficulty, botulinum toxin type A (BTx-A) injections can be combined with non-pharmacologic interventions such as intensive rehabilitation using a robotic approach. To the best of our knowledge, no comparisons have been made between the efficacy of robotic gait training and conventional physical therapy in combination with BTx-A injections.
Objective
To conduct a randomized controlled trial to compare the efficacy on gait of robotic gait training versus conventional physiotherapy after BTx-A injection into the spastic triceps surae in people after stroke.
Method
Thirty-three participants in the chronic stroke phase with triceps surae spasticity inducing gait impairment were included. After BTx-A injection, participants were randomized into 2 groups. Group A underwent robotic gait training (Lokomat®) for 2 weeks, followed by conventional physiotherapy for 2 weeks (n = 15) and Group B underwent the same treatment in reverse order (n = 18). The efficacy of these methods was tested using the 6-minute walk test (6MWT), comparing post-test 1 and post-test 2 with the pre-test.
Results
After the first period, the 6MWT increased significantly more in Group A than in Group B: the mean difference between the interventions was 33 m (95%CI 9; 58 p = 0.007; g = 0.95), in favor of Group A; after the second period, the 6MWT increased in both groups, but the 30 m difference between the groups still remained (95%CI 5; 55 p = 0.019; g = 0.73).
Conclusion
Two weeks of robotic gait training performed 2 weeks after BTx-A injections improved walking performance more than conventional physiotherapy. Large-scale studies are now required on the timing of robotic rehabilitation after BTx-A injection.
Introduction
Stroke is a major cause of death and disability in adults worldwide [1]. It results in spastic paresis which leads to motor impairment. Among survivors, 65% to 85% are able to walk during the first 6 months after stroke, but the functional limitations which often persist impact their quality of life [2,3]. Improving walking ability is one of the main goals of rehabilitation after stroke [4,5]. Both pharmacological and non-pharmacological treatments could be used to reduce the activity limitation caused by spastic paresis [6,7].
Intramuscular injection of botulinum toxin type A (BTx-A) performed under electromyographic or ultrasound guidance has proved to be a safe and effective means of pharmacologically reducing spasticity locally (Grade A according to the French National Health Agency). When lower limb spasticity increases individuals’ walking difficulties, BTx-A can be combined with non-pharmacological interventions as part of a multidisciplinary rehabilitation program to optimize individuals’ walking abilities [8]. Intensive rehabilitation using repetitive task training methods has been found to improve walking performance [4,9]. Robotic gait training (RGT) can be used as a repetitive, intensive, single-task method of gait training with body weight unloading and variable motor assistance, resulting in longer walking times at higher speeds [10], [11], [12]. In an international group consensus on interventions to optimize the benefits of BTx-A after stroke, Francisco et al. wrote that since repetition is important in rehabilitation, robotics are likely to gain more importance in the future [7].
Two studies have addressed the effects of combined treatment with BTx-A and robotic gait training in individuals in the chronic post-stroke phase. Picelli et al. compared the effects on spasticity of combined treatment with BTx-A and RGT with a Lokomat versus BTx-A injection alone [13]. The results showed that RGT did not enhance the antispastic effect of BTx‑A, as measured using the modified Ashworth scale and the Tardieu scale. Interestingly the latter author reported the occurrence of a significant improvement in the distance covered in the 6-minute walk test after combined treatment versus BTx-A alone. Erbil et al. observed greater benefits in terms of individuals’ walking and balance abilities after treatment with physical therapy and RGT with the Robogait as compared with physical therapy alone in individuals in the chronic post-stroke phase previously injected with BTx-A [14].
It was proposed here to test the hypothesis that RGT performed after injection of BTx-A into the triceps surae muscle would improve the walking performance of participants in the chronic post-stroke phase with spastic hemiparesis more than conventional physiotherapy (CP). The chronic phase was chosen to avoid fluctuations in performance caused by natural recovery during the first 6 months post stroke. The second argument for choosing the chronic phase was to reduce the contribution of balance disorders to walking disorders and to increase the contribution of intra-limb coordination and propulsion, which can be trained using a robotic exoskeleton [15,16].
To our knowledge, this is the first comparison of the effects of RGT and CP after BTx-A injection on gait performance in participants in the chronic post-stroke phase. The aims of the present study were as follows:
- -To compare the efficacy of RGT using the Lokomat with that of conventional physiotherapy after triceps surae BTx-A injection in participants with chronic stroke sequelae
- -To assess whether the time elapsed between BTx-A injection and RGT may contribute significantly to the gait improvements observed.
Section snippets
Materials and methods
This manuscript follows the 2017 CONSORT guidelines for reporting randomized trials evaluating non-pharmacological treatments [17].
Results
The flowchart of the results obtained is presented in Fig. 2. A total of 34 participants were enrolled and randomized into 2 groups from February 2019 to May 2021. One participant from Group A was excluded from the analysis because the investigator realized at the end of the assessments that he had been included by mistake. The analysis was based on 15 and 18 participants in Group A and Group B, respectively (Fig. 2). No significant differences were observed between the 2 groups (see Table 1).
Discussion
The aim of this study was to assess the effects of RGT treatment using a fixed exoskeleton combined with BTx-A on the walking performances of people in the chronic post-stroke phase, in comparison with CP combined with BTx-A. To our knowledge, no previous studies have addressed the question of the timing of RGT after botulinum toxin injection. The results presented here show that between W4 and W0, participants’ performances improved in the 6MWT tests more clearly after undergoing RGT than CP.
Conclusion
Our results support the hypothesis that 2 weeks of RGT with a fixed exoskeleton performed 2 weeks after BTx-A improved the walking ability of individuals in the chronic post-stroke phase more than CP. Participants with a spontaneous gait speed between 0.4 and 0.8 m/s appeared to benefit most from RGT performed between W2 and W4 after BTx-A injection. Further studies with groups paired on the basis of gait speed are now required in order to confirm this finding.
Declaration of conflicts of interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Jean Michel VITON reports financial support was provided by French government ministry of health (PHRC). Jean Michel Viton reports a relationship with Allergan France and Merz Pharma that includes support for attending meetings and travel reimbursement. Maëva COTINAT reports a relationship with Ipsen Pharma and Allergan France that includes support for attending
Funding
This study was sponsored by Assistance Publique – Hôpitaux de Marseille (DRCI).
Acknowledgement
The authors thank the participants in this study and the rehabilitation teams for their invaluable assistance, Pr. Julien Mancini for his help with statistics and Mrs. Jessica Blanc for her English writing correction.
References (34)
- P. Langhorne et al.Stroke rehabilitationLancet(2011)
- E. Allart et al.Adjunct therapies after botulinum toxin injections in spastic adults: systematic review and SOFMER recommendationsAnn Phys Rehabil Med(2022)
- G. Moucheboeuf et al.Effects of robotic gait training after stroke: a meta-analysisAnn Phys Rehabil Med(2020)
- T. Veverka et al.Cortical activity modulation by botulinum toxin type A in patients with post-stroke arm spasticity: real and imagined hand movementJ Neurol Sci(2014)
- C. Delcamp et al.Botulinum toxin combined with rehabilitation decrease corticomuscular coherence in stroke patientsClin Neurophysiol(2022)
- S.Y. Shin et al.Soft robotic exosuit augmented high intensity gait training on stroke survivors: a pilot studyJ NeuroEngineering Rehabil(2022)
- H. Hsiao et al.Control of lateral weight transfer is associated with walking speed in individuals post-strokeJ Biomech(2017)
- Johnson CO et al.Global, regional, and national burden of stroke, 1990–2016: a systematic analysis for the global burden of disease study 2016Lancet Neurol(2019)
- J. Eng et al.Gait training strategies to optimize walking ability in people with stroke: a synthesis of the evidenceExpert Rev Neurother(2007)
- D. Wade et al.Walking after stroke. Measurement and recovery over the first 3 monthsScand J Rehabil Med(1987)
View more references
[ARTICLE] Can the early use of botulinum toxin in post stroke spasticity reduce contracture development? A randomised controlled trial – Full Text
Posted by Kostas Pantremenos in Pharmacological, Spasticity on November 24, 2022
Abstract
Objective:
Does early treatment of spasticity with botulinum-toxin (BoNTA), in (hyper)acute stroke patients without arm-function, reduce contractures and improve function.
Design:
Randomised placebo-controlled-trial
Setting:
Specialised stroke-unit.
Participants & Intervention:
Patients with an Action Research Arm Test (ARAT) grasp-score⩽2 who developed spasticity within six-weeks of a first stroke were randomised to receive injections of: 0.9%sodium-chloride solution (placebo) or onabotulinumtoxin-A (treatment).
Outcome-Measures:
Spasticity, contractures, splint use and arm function (ARAT) were taken at baseline, 12-weeks post-injection and six-months after stroke. Additionally, spasticity and contractures were measured at weeks-two, four and six post-injection.
Results:
Ninety three patients were randomised. Mean time to intervention was 18-days (standard deviation = 9.3). Spasticity was lower in the treatment group with difference being significant between week-2 to 12 (elbow) and week-2 to 6 (wrist). Mean-difference (MD) varied between –8.5(95% CI –17 to 0) to –9.4(95% CI –14 to –5) µV.
Contracture formation was slower in the treatment group. Passive range of motion was higher in the treatment group and was significant at week-12 (elbow MD6.6 (95% CI –0.7 to –12.6)) and week-6 (wrist MD11.8 (95% CI 3.8 to 19.8)). The use of splints was lower in the treatment group odds ratio was 7.2 (95% CI 1.5 to 34.1) and 4.2 (95% CI 1.3 to 14.0) at week-12 and month-6 respectively.
Arm-function was not significantly different between the groups MD2.4 (95% CI –5.3 to 10.1) and 2.9 (95% CI –5.8 to 11.6) at week-12 and month-6 respectively.
Conclusion:
BoNTA reduced spasticity and contractures after stroke and effects lasted for approximately 12-weeks. BoNTA reduced the need for concomitant contracture treatment and did not interfere with recovery of arm function.
Introduction
Recovery of arm function in people who survive a stroke is commensurate with severity of impairment at stroke onset.1 Those people who have severe impairment of the arm at onset and who do not recover useful arm function, are likely to develop contractures.2,3 Contracture formation is exacerbated in those who have certain forms of spasticity.4–6 It can therefore be hypothesised that the lack of movement (as a result of the paralysis) in addition to the fixed positioning (associated with some forms of spasticity) accelerate the formation of contractures.6
Contractures are characterised by the combination of increased stiffness and loss of range of movement at a joint. Contractures can be established within four weeks of a stroke and 52% of stroke survivors have developed a contracture at six months.4,7,8 In some cases where motor recovery is delayed, it is possible that contractures could limit the recovery of meaningful function rather than the lack of neuro-plastic potential.
One way of slowing contracture development is through intensive mobilisation using cyclical electrical stimulation and this has been demonstrated previously in stroke patients at risk of wrist flexion contractures.9 The aim of this study was to explore if preventing the fixed positioning associated with spasticity (using additional treatment with botulinum toxin) could reduce both contractures and the rate at which contractures were formed. It was hypothesised that the reduction of spasticity and contractures would lead to a subsequent improvement in arm function. This study, therefore, also aimed to quantify changes in arm function following treatment. […]
[Abstract] A systematic review on extracorporeal shock wave therapy and botulinum toxin for spasticity treatment: a comparison on efficacy
Posted by Kostas Pantremenos in Spasticity on April 28, 2022
INTRODUCTION: The complexity of spasticity requires a continuous effort in terms of more adapted treatments for patients and accurate management. Through this systematic review, we aimed to evaluate and compare the effectiveness of Extracorporeal Shock Wave Therapy (ESWT) with Botulinum Toxin Type A (BoNT-A) on reducing spasticity both in children and adults.
EVIDENCE ACQUISITION: An electronic search of PubMed/ MEDLINE, Scopus, Ovid MEDLINE(R), and search engine of Google Scholar was performed. Publications ranging from January 2010 to January 2021, published in the English language and available as full-texts were eligible for inclusion and they were searched without any country restriction. The study was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines.
EVIDENCE SYNTHESIS: A total of five studies were included in the present systematic review. Screening of the references, data extraction, and risk of bias assessment were performed by two independent authors. The methodological quality and risk of bias were conducted using the Physiotherapy Evidence Database (PEDro) scale. The primary outcome was spasticity grade assessed by the Modified Ashworth Scale (MAS) and/or Modified Tardieu Scale (MTS). Additional outcomes were active range of motion (AROM), passive range of motion (PROM), upper extremity Fugl-Meyer Assessment (UE-FMA), pain intensity assessed through Visual Analogue Scale (VAS), spasm frequency scale (SFS), sonographic parameters, between-group comparison, and treatment response rate.
CONCLUSIONS: A beneficial effect on spasticity was found for both treatments: evidence showed that ESWT and BoNT-A can ameliorate spasticity considering parameters such as MAS, MTS, AROM, PROM, UE-FMA, VAS and SFS in post-stroke, multiple sclerosis, and cerebral palsy patients. Further research is required to strengthen the evidence, and more suitable study protocols are highly needed.
[ARTICLE] Constraint-Induced Movement Therapy Combined With Botulinum Toxin for Post-stroke Spasticity: A Systematic Review and Meta-Analysis – Full Text
Posted by Kostas Pantremenos in Constraint induced movement therapy CIMT, Neuroplasticity, Spasticity on March 9, 2022
Abstract
Stroke is considered one of the main causes of adult disability and the second most serious cause of death worldwide. The combination of botulinum toxin type A (BTX) with rehabilitation techniques such as modified constraint-induced movement therapy (mCIMT) has emerged as a highly efficient intervention for stroke patients to start synchronized motor function along with spasticity reduction. The current systematic review and meta-analysis were conducted in order to evaluate the available literature about the safety and efficacy of constraint-induced movement therapy (CIMT) combined with BTX in stroke patients with upper limb spasticity.
Searches were conducted on WoS (Web of Science), Ovid, EBSCO-ASC&BSC, and PubMed for identifying relevant literature published from 2000-2020. Randomized Controlled Trials (RCTs) and Quasi-experimental studies were considered for inclusion. Rayyan (systematic review tool) QCRI (Qatar Computing Research Institute) was used for independent screening of the studies by two reviewers. For risk of bias and study quality assessment, Cochrane risk of bias tool (RoB 2) and Physiotherapy Evidence Database (PEDro) scales were used. Cochrane review manager was used to carry out the meta-analyses of the included studies.
The search resulted in a total of 13065 references, of which 4967 were duplicates. After the title, abstract and full-text screening, two RCTs were deemed eligible for inclusion. Both the RCTs scored 8 on PEDro and were level evidence. The studies were heterogeneous. The findings of this meta-analysis in all the three joints post-stroke spasticity assessed on modified Ashworth scale (MAS) at four weeks post-injection aren’t statistically significant (elbow P-value 0.74, wrist P-value 0.57, fingers P-value 0.42), however, according to one of the included studies the therapeutic efficacy of the combination of BTX-mCIMT injection assessed at four weeks post-injection in wrist and finger flexors was promising.
The effectiveness of BTX-CIMT combination over conventional therapy (CT) for improving post-stroke spasticity still needs to be explored with long-term, multicenter rigorously designed RCTs having a good sample size. However, the BTX-CIMT combination is promising for enhancing motor function recovery and improving activities of daily living (ADLs).
Introduction and background
Stroke is among the most serious diseases globally. It affects millions of people all over the world. It is associated with a high economic burden every year due to rehabilitation and pain [1,2]. Consequently, it is not only affecting the economies of various countries but also lowering the quality of life of their populations [1]. Over the past few decades, multiple treatment strategies were developed to alleviate this health problem, including rehabilitation and drug therapy.
Rehabilitation techniques include but are not limited to mirror therapy, virtual reality, robot-assisted rehabilitation and constraint-induced movement therapy (CIMT) [3–5]. CIMT represents a high-intensity and task-specific training after stroke, particularly for the upper extremities. Nevertheless, the broad adoption of CIMT post-stroke is hindered by the presence of significant spasticity [6,7].
Spasticity, a common complication of stroke, hinders the rehabilitation process substantially and requires practical and effective management [8]. Its management may include non-pharmacological treatment modalities such as shock waves therapy and pharmacotherapy with oral anti-spasticity drugs such as baclofen, dantrolene, benzodiazepines, or phenol and alcohol injections [9–13]. However, some medications are frequently accompanied by side effects such as muscle weakness and fatigue [14].
A highly promising therapeutic approach to post-stroke spasticity was the injection of botulinum toxin type A (BTX), which is associated with minor side effects. Although the effect of BTX injections in combination with CIMT showed a promising impact on arm motor function and reduced spasticity in a chronic stroke patient, this combination has not been fully elucidated thus far [15].
The combination of BTX injections and CIMT (BTX-CIMT) is possibly a promising approach for such an important issue. These injections can counterbalance the arms’ motor recovery along with managing the spasticity [16]. Besides, the use of BTX-CIMT in upper limb post-stroke spasticity management has resulted in significant improvements in the capacity and performance of the affected upper limb [15, 17].
This meta-analysis aimed to evaluate the available literature about the safety and efficacy of CIMT combined with BTX in stroke patients with upper limb spasticity. This will keep researchers, decision-makers, and healthcare practitioners up-to-date with the current evidence on the topic.
[WEB] Stroke Spasticity: What It Is, How to Manage, and More.
Posted by Kostas Pantremenos in Spasticity on May 5, 2021
- Post-stroke spasticity can make it difficult to stretch, move, and accomplish everyday tasks.
- Modifying your home, working with an occupational therapist, practicing daily exercises, and using mobility aids can help you manage spasticity.
- Treatments, such as injections and medications, can help reduce long-term damage from spasticity.
Strokes occur when blood flow to the arteries in the brain become blocked, or (in more serious cases) leak or burst. This causes trauma to the brain and spinal cord, which can lead to other symptoms.
Between 25 percent and 43 percent of people will experience a condition called spasticity in the first year after a stroke, according to the American Stroke Association.
Spasticity causes muscles to become stiff and tight, making it difficult to stretch, move, and take care of everyday tasks.
Fortunately, treatments and lifestyle adjustments can help reduce the severity of the condition and its impact on your life.
Read on to learn more about spasticity and ways to manage it.
What is spasticity after a stroke?
A stroke can damage the part of the brain that controls the signals to the muscles. If that happens, you may experience spasticity, or an abnormal increase in muscle tone.
It can cause your muscles to get stiff, tight, and painful, causing you to be unable to move fluidly.
That, in turn, can affect the way you speak, move, and walk. Your muscles may remain contracted in certain positions, like a bent wrist, clenched fist, or tucking your thumb into your palm, according to the American Association of Neurological Surgeons.
Other ways spasticity can affect the body after a stroke include:
- tight knees
- tension in the fingers
- bending your foot at an angle
- weakness in a foot, causing it to drag when walking
- bending your arm and holding it tight against the chest
- curling in the toes
Spasticity tends to be more common in younger people who have a stroke, according to the American Stroke Association. Strokes that are caused by a bleed can also increase the risk of spasticity.
How is it treated?
Treatment options for spasticity after a stroke depend on the severity of your symptoms. Your doctor may also suggest trying a variety of treatments and management strategies at the same time.
Here are some common treatment options, according to the American Stroke Association:
- exercise and stretching
- muscle braces
- injections of certain medications, such botulinum toxin (Botox)
- oral medications, such as baclofen, diazepam, tizanidine, and dantrolene sodium
- intrathecal baclofen therapy (ITB)
There are also lifestyle changes people can make to reduce the symptoms of spasticity after a stroke.
How to manage spasticity after a stroke
While spasticity can be painful, there are ways to reduce symptoms of the condition and improve your quality of life.
Here are seven tips for living with spasticity:
1. Exercise or stretch the affected limbs
One of the best things you can do for spasticity after a stroke is to keep the affected limbs moving.
Regularly exercising these areas can help ease tightness, prevent muscles from shortening, and maintain your full range of motion.
A physical therapist or occupational therapist can show you exercises that may help your post-stroke spasticity.
2. Adjust your posture
Try to avoid staying in one position too long if you’re coping with spasticity after a stroke. That can cause muscles and joints to get stiff and sore.
Caregivers should aim to help people with spasticity switch positions every 1–2 hours to help keep the body limber.
3. Support affected limbs
Providing extra support for affected limbs can also keep you more comfortable and reduce the effects of spasticity. For example, try not to let your arm or leg fall off the side of the bed or wheelchair.
Be especially mindful when lying down. Placing your affected arm or leg under your body when resting can worsen spasticity.
Lying on your back can help keep your limbs in a more comfortable position. If you prefer to lie on your side, avoid putting the weight on the side that the stroke affected.
Special braces can help support limbs and prevent spasticity from getting worse.
4. Adapt your home
Making adjustments around the home can make it easier for people with spasticity to move around and accomplish tasks.
Here are some ways you can adapt your home, according to the American Stroke Association:
- install ramps to doorways
- add grab bars to the bathroom
- install raised toilet seats
- place a bench in your tub or shower
- use plastic adhesive strips on the bottom of your tub
5. Ask for support
People with spasticity, along with their caregivers, can find it helpful to seek support from family, friends, and other loved ones. They can encourage active movement and help with tasks around the home.
It can also be a great way to bond and enjoy time together. If your loved one is stretching, for instance, try stretching with them for encouragement.
6. Work with an occupational therapist
Occupational therapists help people with disabilities and health conditions learn new ways of performing everyday tasks more easily.
This may mean learning to get dressed with the opposite hand, or modifying eating habits. While learning something new is always a journey, staying positive can help make the process easier.
7. Use mobility aids
If spasticity has made it difficult to get around after a stroke, using mobility aids can help you move more easily. Common mobility aids include:
- braces
- wheelchairs
- canes
- walkers
Talk with an occupational therapist to see if a mobility aid can be helpful for you.
Does stroke spasticity go away and how long can it last?
Spasticity often occurs between 3 and 6 weeks after a stroke, according to research from 2018. The muscular symptoms of spasticity have been shown to continue increasing at 6 months after a stroke.
If left untreated, spasticity can cause permanent shrinking and contracting of the muscles, along with joints locked into single positions.
While there’s no cure for post-stroke spasticity, treatments and lifestyle changes can help reduce symptoms and maintain your range of motion.
The takeaway
At least a quarter of people will develop spasticity after a stroke. The condition can cause tight, stiff muscles and reduce your mobility.
You can manage symptoms and improve your quality of life with spasticity by modifying your home, practicing daily exercises, working with an occupational therapist, and using mobility aids.
Treatments can also help prevent long-term damage from spasticity. Talk with a doctor to see if medication or injections are right for you.
[ARTICLE] Goal attainment: a clinically meaningful measure of success of Botulinum Toxin-A treatment for lower limb spasticity in ambulatory patients – Full Text
Posted by Kostas Pantremenos in Pharmacological, Spasticity on April 24, 2021
Abstract
Objective
The objectives of this study were: (1) to evaluate whether Botulinum toxin type A (BoNT-A) treatment for lower limb spasticity leads to patient goal attainment and identify factors associated with positive goal attainment, and (2) to assess the effect of BoNT-A treatment on patients’ gait.
Design
Retrospective cohort study between June 2014 and February 2019.
Setting
Public outpatient spasticity clinic in a tertiary hospital.
Participants
Thirty patients (50% female, average age 50.5 years) with lower limb spasticity of heterogenous aetiologies (96.7% cerebral ± spinal origin and 3.3% isolated spinal origin). 73.3% of patients had previously received BoNT-A treatment.
Interventions
BoNT-A injection to lower limb muscles.
Main outcome measures
The primary outcome measure was goal attainment measured using the Goal Attainment Scale (GAS). The Modified Ashworth Scale (MAS) was used to assess spasticity. Gait was characterised by spatiotemporal parameters.
Results
Fifty-six treatment episodes were analysed and showed BoNT-A treatment resulted in a significant reduction in spasticity (pre-treatment MAS = 3.18±0.73; post-treatment MAS = 2.27±0.89, p<0.001) with no associated change in gait parameters. Logistic regression revealed most patients (74.1%) achieved all of their goals with younger patients having a high likelihood of goal attainment regardless of their gait profile identified by latent profile analysis of the gait parameters. Patients considered to have a low functioning gait profile demonstrated a significantly greater likelihood of goal attainment than the patients of the other gait profiles combined (OR= 45.6, 95% CI= 1.3 to 1602.1; p=0.036). Chronic spasticity, pre-treatment severity of spasticity (MAS) and its reduction were not associated with likelihood of goal attainment.
Conclusion
The success and efficacy of BoNT-A treatment in improving patient perceived gait quality and reducing the negative symptoms of spasticity was best measured using the GAS. The study emphasises the importance of measuring patient goals as a clinical outcome. Gait parameters were most informative when used collectively to classify patients on the basis of their overall gait profile which assisted in identifying differences between patients’ likelihood of goal attainment following treatment.
——————————-
Spasticity, a sequelae of numerous neurological disorders, is characterised by a velocity dependent increase in muscle tone that results in resistance to passive movement, involuntary muscle spasms and contractions (1, 2). Lower limb spasticity can result in disabling consequences including pain, spasm, altered posture, deformity of the foot and ankle, and impairment of gait and mobility (3, 4). The impact on gait and mobility is associated with loss of function and independence, higher morbidity including falls and fracture (3) and premature residential aged care placement (5-7). Prevention and management of lower limb spasticity and sequelae is therefore an important focus of neurological rehabilitation.
Previous research has demonstrated the positive effects of focal injections of the neurotoxic protein botulinum toxin A (BoNT-A) in treating spasticity and it is now a widely accepted treatment modality (8-18). Studies investigating the effect of BoNT-A on lower limb spasticity have concentrated on outcomes including gait, safe and independent mobility, and activities of daily living (3, 8-10, 13-15, 17, 19-27). To date, the evidence regarding the benefit of BoNT-A mediated reduction in lower limb spasticity on functional outcomes remains inconsistent (20).
In clinical practice the indications and objectives for BoNT-A treatment of lower limb spasticity are diverse and patient specific, as are the patient’s priorities and expectations of the treatment. Rehabilitation-centred frameworks should therefore include a meaningful patient focused purpose for BoNT-A treatment, beyond reducing spasticity itself (28), by identifying patient needs, priorities and goals and tailoring treatment towards addressing and achieving these.
Few previous studies examining BoNT-A treatment for lower limb spasticity have reported the nature of patient goals, examined goal attainment outcomes or investigated factors associated with the likelihood of goal attainment (15, 29-31). A better understanding of such relationships is of clinical value, may guide patient selection and help predict positive treatment outcomes. Hence, the primary aim of this study was to evaluate the attainment of patients’ self-identified treatment goals, and factors associated with the likelihood of patient goal attainment. A secondary aim was to assess the effect of BoNT-A treatment on the gait of patients with lower limb spasticity.[…]
[Abstract] Combination therapy with Repetitive Facilitative Exercise Program and Botulinum Toxin Type A to improve motor function for the upper-limb spastic paresis in Chronic Stroke: A Randomized Controlled Trial
Posted by Kostas Pantremenos in Paretic Hand, Pharmacological, Spasticity on January 29, 2021
Highlights
- Forty chronic stroke patients with upper-limb spastic paresis were enrolled an RCT
- Control group (CG) received repetitive facilitative exercise (RFE) program only.
- Intervention group (IG) received BoNT-A injection combined with the RFE program.
- Motor control and motor functions were evaluated during 4-week study period.
- IG evidenced significantly greater improvement in the outcome measures than CG.
Abstract
Study Design
An open-label, randomized, controlled, observer-blinded trial.
Introduction
Repetitive facilitative exercise (RFE) is a movement therapy to recover from hemiparesis after stroke. However, improvement is inhibited by spasticity. Recently, botulinum toxin type A (BoNT-A) injection has been shown to reduce spasticity.
Purpose
To examine the combined effect of an RFE program and BoNT-A treatment on upper-limb spastic paresis in chronic stroke.
Methods
Forty chronic stroke inpatients with upper-limb spastic paresis (Brunnstrom stage ≥III and Modified Ashworth Scale (MAS) score ≥1) were enrolled. Subjects were randomized into two groups of 20 each and received 4 weeks of treatment. The intervention group received RFE and BoNT-A injection; the control group underwent RFE only. Assessments were performed at baseline and at study conclusion. The primary outcome was change in Fugl–Meyer Assessment score for the upper extremity (FMA). The Action Research Arm Test (ARAT), active range of motion, Box and Block Test, and MAS were also evaluated.
Results
All participants completed this study. After 4 weeks, the intervention group evidenced a significantly greater increase in FMA score [median 11.0 (range 4 to 20)] than the control group [median 3.0 (range 0 to 9)] (p<0.01, r=0.79); as well as improvements in the other measures such as ARAT [median 12.5 (range 4 to 22) vs. 7 (0 to 13)] (p<0.01, r=0.6), and MAS in the elbow flexors [median −1.5 (range −2 to 0) vs. −1 (−2 to 0)] (p<0.01, r=0.45).
Discussion
A high degree of repetitive volitional movement induced by the facilitative technique with concomitant control of spasticity by BoNT-A injection might increase efficiency of motor learning with continuous movement of the affected upper-limb.
Conclusions
The combination of RFE and BoNT-A for spastic paresis might be more effective than RFE alone to improve upper-limb motor function and to lessen impairment in chronic stroke.
[Abstract] Self-rehabilitation combined with botulinum toxin to improve arm function in people with chronic stroke. A randomized controlled trial
Posted by Kostas Pantremenos in Paretic Hand, Pharmacological, REHABILITATION, Tele/Home Rehabilitation on November 10, 2020
Abstract
BACKGROUND:Botulinum toxin injection (BTI) reduces muscle hyperactivity but its effect on active upper limb function is limited. Intensive rehabilitation could optimize the effects; however, outpatient post-stroke rehabilitation is usually not intensive. One solution could be self-rehabilitation. OBJECTIVES:The aim of this randomized controlled trial was to determine the effects of a self-rehabilitation program combined with BTI on upper limb function in individuals with chronic hemiparesis. METHODS:Thirty-three outpatients were randomly allocated to receive either BTI + self-rehabilitation (R group: n=17) or BTI alone (C group: n=16). Outcomes evaluated just before the BTI and 4 weeks later included: Wolf Motor Function Test (WMFT time – Primary Outcome), Action Research Arm Test, fatigue and quality of life. RESULTS:Change in WMFT was not different between groups at 4 weeks (WMFT time: -14% for R group, -4 % for C group. WFMT score: +12 % for R group, 0 % in C group). WFMT time and score improved significantly (-14 %, p=0.01 and +12 %, p=0.02 respectively) in the R group only. In addition, the proportion of patients with improved WMFT time and scores was higher in the R group (71% improved score, 77% improved time, C group 43% improved score, 50% improved time). Passive range of shoulder flexion (p=0.03) and wrist extension (p=0.01) also improved only in the R group. No other variables changed significantly. Compliance was excellent; average daily training time was greater than that prescribed. CONCLUSIONS:The addition of a self-rehabilitation program to BTI did not significantly improve functional outcomes more than BTI alone, however movement quality and speed only improved in the self-rehabilitation group. Participants in the self-rehabilitation group trained more than they were asked to, suggesting they found the program worthwhile. These clinically relevant findings justify larger-scale studies into the effects of self-rehabilitation to enhance the effects of BTI.
[Abstract] Improvement of active movement and function in adults with chronic spastic paresis following repeated treatment with abobotulinumtoxinA (Dysport®)
Posted by Kostas Pantremenos in Paretic Hand, Pharmacological, Spasticity on October 20, 2020
Objective
There are limited data on improvements of active limb movement and function following treatment with botulinum toxin in chronic spastic paresis. We report the effect of repeated injections of abobotulinumtoxinA (aboBoNT-A) on these parameters from two Phase III multicenter open-label (OL) trials in adults with spasticity post-stroke/traumatic brain injury; one trial in upper limb spasticity, the other in lower limb spasticity. These are extensions to respective double-blind studies (DB) in which adults received a single aboBoNT-A injection (Gracies et al. Lancet Neurol 2015; Esquenazi et al. AAPM&R 2016).
Material/Patients and methods
Subjects (18–78 years) received aboBoNT-A (500 to 1500U) over a year (injections ≥ 12 weeks apart) in their affected limb. Active movement was assessed by active range of motion (XA) against elbow, wrist and finger flexors or active ankle dorsiflexion. Active function was assessed by Modified Frenchay Scale (MFS) (upper limb) or the 10-meter walking speed test (lower limb). Results for cycle 4 week 4 of the OL phase are presented.
Results
Eighty-one subjects received 5 injections in their UL and 139 subjects in their LL. XA improved in the upper limb across injection cycles, with active finger extension (most frequently injected muscle group) increasing by a mean (SD) of +38.0 (53.4)°. The overall increase in MFS was +0.40 (0.75), an improvement that was more pronounced with 1500U (500U in shoulder muscles): +0.62 (0.48) vs. +0.30 (0.83) for 1000U. Active ankle dorsiflexion improved by +6.5(10.9)° with knee extended. Comfortable walking speed improved by +0.088 (0.144) (mean increase of 25% from baseline of DB phase).
Discussion/Conclusion
Improvement in active movement and function in subjects with chronic upper or lower limb spasticity was observed following repeat injections of aboBoNT-A over a year. A more pronounced efficacy with 1500U versus 1000U aboBoNT-A for active function in the upper limb may suggest the importance of shoulder muscle injections.
[ARTICLE] Ankle and Foot Spasticity Patterns in Chronic Stroke Survivors with Abnormal Gait – Full Text
Posted by Kostas Pantremenos in Gait Rehabilitation - Foot Drop, Spasticity on October 14, 2020
Abstract
Chronic stroke survivors with spastic hemiplegia have various clinical presentations of ankle and foot muscle spasticity patterns. They are mechanical consequences of interactions between spasticity and weakness of surrounding muscles during walking. Four common ankle and foot spasticity patterns are described and discussed through sample cases. The patterns discussed are equinus, varus, equinovarus, and striatal toe deformities. Spasticity of the primary muscle(s) for each deformity is identified. However, it is emphasized that clinical presentation depends on the severity of spasticity and weakness of these muscles and their interactions. Careful and thorough clinical assessment of the ankle and foot deformities is needed to determine the primary cause of each deformity. An understanding of common ankle and foot spasticity patterns can help guide clinical assessment and selection of target spastic muscles for botulinum toxin injection or nerve block.
1. Introduction
About 80% of chronic stroke survivors have varying degrees of abnormal gait and impaired locomotion capability [1]. Spasticity in the ankle and foot muscles is very common, and often results in various ankle and foot deformities, including equinus, varus, equinovarus, and striatal toe deformities. The spastic equinovarus deformity is the most common deformity seen [2,3,4]. An ankle and foot joint abnormality could have subsequent effects on the knee, hip, and trunk position and control in post-stroke hemiplegic gait through a kinetic chain effect. For example, an equinovarus deformity shifts the ground reaction force anterior to the knee joint, and thus facilitates knee (hyper)extension during the stance phase. Stroke survivors are often forced to increase hip extension to compensate for knee (hyper)extension to keep the center of gravity within the forefoot. During the swing phase, increased knee and hip flexion is required to clear the equinovarus foot from the floor. However, they are often unable to do so due to weak hip and knee flexors, and, instead, present with hip hiking and leg circumduction. Additionally, stroke survivors have a smaller base of support due to the equinovarus deformity. The stance phase is shortened to minimize the risk of fall. Therefore, the ankle and foot deformity is often associated with kinetic and kinematic gait abnormalities, such as gait asymmetry, slow speed, genu recurvatum, etc [3,5].Joint abnormalities in the hip, knee, ankle, and foot joints observed in post-stroke hemiplegic gait are mechanical consequences of the interactions among muscle spasticity, weakness, and disordered motor control during locomotion [6]. Depending on the severity of spasticity and weakness of muscles surrounding a joint, various joint abnormalities can develop. The complex ankle and foot anatomy contribute directly to observed deformities. As shown in Figure 1, four groups of muscles (invertors, evertors, dorsiflexors, and plantarflexors) act on the ankle–foot complex. Any isolated ankle movement is a net result of the combined activation of a group of target muscles, e.g., inversion occurs when dorsiflexors (primarily the tibialis anterior muscle) and plantarflexors (primarily the tibialis posterior muscle) co-activate. In the presence of spasticity, stroke survivors have less control and isolated activation; activation is more diffuse and divergent [7,8]. Therefore, a variety of ankle–foot deformities could be observed, depending on the severity of spasticity and weakness of individual muscles.
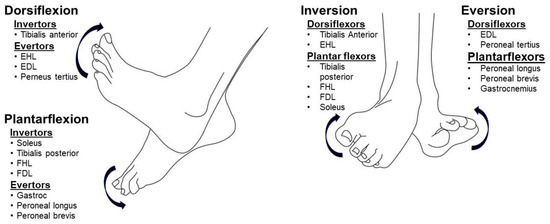
Among the spectrum of treatment options for ankle and foot deformities and gait disorders, interventions such as botulinum toxin (BoNT) injection and phenol neurolysis are commonly used to manage spasticity of the ankle and foot muscles [2,4,11,12]. To achieve the best clinical outcomes, it is important to identify the primary causes of the deformity. Based on the assessment from instrumented gait analysis, BoNT treatment for spasticity of target muscles has shown to improve gait pattern and walking speed [13,14,15]. However, an instrumented gait analysis lab is not available in most clinics and it is not practical to perform a computer assisted gait analysis for every patient. Understanding common ankle and foot spasticity patterns is helpful to guide our clinical assessment and development of a treatment plan. These common ankle and foot spasticity patterns are presented here through sample cases. In all cases, no significant component of contracture was detected. The common ankle and foot spasticity patterns include: equinus, varus, equinovarus, and striatal toe.[…]
