Archive for February, 2023
[WEB] Putting your best foot forward – Why now is the time for adaptive footwear
Posted by Kostas Pantremenos in Assistive Technology, Gait Rehabilitation - Foot Drop on February 27, 2023

Joseph DiFrancisco, Occupational Therapist and Founder of Friendly Shoes, discusses the growing adaptive footwear market.
Life requires footwear, and putting on shoes is among the most essential activities of daily living. Yet, as a practicing occupational therapist, I witnessed a huge range of individuals who struggled putting on and fitting the shoes they want and need to flourish.
Until a certain age, even the most precocious children struggle with footwear. On the other end of the spectrum, virtually all older adults will struggle with footwear even without a distinct physical or neurological condition. In between, a huge portion of us will experience injuries, accidents, or adverse diagnoses, the effects of which are likely to include difficulty performing putting on our shoes.
The shoe industry’s response historically has been the loathsome “old man” shoe: oversized, over-width, velcro, single colour, BORING. Even if my patients could put these on, they typically did not fit well and they surely did not want to wear them. Furthermore, the mere realisation that the shoes were “there” diminished self-concept.
I saw the need for attractive, accessible footwear for this divergent population, but it didn’t exist. So, in 2014 I took a hacksaw to a trainer trying to help a single patient access his work shoes so he could continue supporting his family. I didn’t know it then, but Friendly Shoes was born that day.
My vision was footwear attractive enough for anyone and accessible to almost everyone. It had to look, feel, and work as well as modern footwear but also accommodate the challenges my patients experienced. I knew it would be a valuable tool for my colleagues to help their patients increase functional independence and quality of life. So, I began building shoes from the minds of therapists for the needs of our patients. I named my company Friendly because friendly means helping others, and that’s what our shoes do.
Common myths about adaptive footwear
Adaptive is for disabilities
The big myth is that adaptive footwear is for disabilities or advanced age users. Reality is that the same features that help accommodate challenges presented by physical, neurological, and age-related conditions can benefit anyone. After all, who really enjoys tying laces multiple times per day or wearing oversized or loosely tied shoes simply to avoid lacing and make donning easier?
Accessible footwear that adapts to user needs or preferences substantially improves over traditional footwear, so long as essential style, comfort, and support are not sacrificed.
Brands, such as Friendly Shoes, that combine 21st century convenience and adaptability with modern style and comfort present compelling advantages that may be attractive to any person. The fact that an able-bodied person may opt for style, comfort, or adaptability – the same shoe that can accommodate donning challenges and orthotics – is what makes it inclusive.
Specialist shoes are unattractive
To be fair, historically, this has been true. Times, however, are changing. While there are only a small number of authentically adaptive footwear companies, including Friendly Shoes, these offer a wide range of styles and colours as attractive as any other brand. As real adaptive companies grow, we will continue to see more styles, widths, and specialty adaptations meeting more patient needs.

Who benefits most from adaptive footwear?
Donning challenges
From a clinician/therapist perspective, individuals experience footwear challenges due to a huge variety of injuries, conditions, or diagnoses. Older adults experience generalised weakness, loss of dexterity, diminished eyesight, and bending difficulties. Numerous neurological challenges, from cerebral palsy to Parkinson’s and strokes, cause tremoring, loss of mobility, and functional endurance challenges.
Fitting challenges
Wide feet, bunions, specialty insoles, orthotics/AFO, intermittent swelling, age-related changes, and variations in foot size rule out the majority of available footwear for huge portions of the population. Footwear that can adjust in width and depth helps accommodate these challenges, and many of these individuals simultaneously experience donning challenges.
Carers/families
Let’s not forget the substantial reduction in carer/family burden from easier footwear and the time and cost savings to facilities by making a tedious task faster and easier. Adaptive footwear helps make a difficult aspect of caregiving easier.
What to look for in adaptive footwear
Effective access technologies: One size does not fit all nor how we put on footwear. Technologies enabling access from multiple positions with different donning strategies accommodate more types of challenges than hands-free gimmicks, which can be unsafe for certain adaptive populations.
Adjustability: Tension opportunities, removable insoles, and free-floating tongues help accommodate differently sized feet, variability in swelling, thicker socks, inserts, and orthotics to provide proper fit.

Lightweight: Many of the medical challenges that make donning and fitting difficult also affect strength and endurance. Lightweight shoes encourage active living and make mobility easier.
Durability: Orthotics are hard on shoes. Toe-drag is a common symptom of numerous conditions. Heavyset individuals place extra strain on footwear. Cheap zippers break. Look for YKK zippers and brands that directly communicate with customers and clinicians to solve problems.
Smooth interiors: If shoes are hard to put on so are socks. Smooth interiors accommodate barefoot use, ensuring that even if an individual can’t put on their sock, they can access appropriate footwear for safe mobility. Smooth interiors are crucial for individuals with hypersensitivity, especially prevalent in autism. Look for brands without interior tags.
Slip resistance: This is a must for safety and stability.
The role of healthcare professionals in adaptive footwear
Adaptive footwear is not a panacea solving all that ails anyone. Adaptive footwear is tools for users and clinicians to accommodate functional challenges. Clinicians play an important role in using their trained, professional lens to work with patients, demonstrating multiple donning techniques, training patients in proper use (e.g. pull zippers back not out), and problem solving on a case-by-case basis to overcome challenges.
We have seen therapists build-up pull tabs, remove insoles, customise lace settings, and, most importantly, train the user in donning strategies from different positions, enabling them to independently don and fit their shoes.
Where do I find adaptive footwear?
When I began the Friendly journey, there were zero adaptive brands and zero adaptive retailers. Today, more and more retailers tout a selection of adaptive products, including footwear. We are proud to have been part of building awareness of the needs of the adaptive community and offering best-in-class adaptive products. Friendly Shoes are available in eight countries.

Moreover, several of our distributors, including in the United Kingdom, are themselves therapists who learned about Friendly Shoes online, immediately recognised how they would benefit their patient communities, and reached out to us requesting to introduce Friendly to their countries. This authentic, grassroots, organic distribution may be slower than million-shoe orders from established retailers, but we value the interest and participation of clinicians more than anything else.
The Friendly Shoes Spring/Summer ’23 collection has just launched, featuring several new colours of popular styles. Our Autumn/Winter ’23 line goes into production shortly.
Please follow our social media in the USA and UK for updates and inspiring stories featuring individuals whose lives are being positively impacted by Friendly Shoes. We value clinician input and welcome your outreach. Thank you for being Friendly.
@friendlyshoesuk
www.friendlyshoes.co.uk
@friendlyshoesus
www.friendlyshoes.com
[WEB] The Benefits of Mindful Movement in Physical Therapy
Posted by Kostas Pantremenos in Uncategorized on February 25, 2023

By Karen Danchalski, PT, DPT
The benefits of mindful movement have been well researched in numerous medical, scientific, and health journals and the research is growing. In fact, it may surprise you just how much research there is out there. As mentioned in the first article of this series, The Case for Mindful Movement in PT, there are over 17,000 abstracts relevant to the study of energy medicine, mindfulness, tai chi, qigong, and yoga in the Qigong Institute database. The institute published a curated collection of these abstracts in their 2022 report, “An Introduction to Qigong Health Care – Meditative Movement Exercise for Whole Person Health,” which can be accessed through the National Qigong Institute.
Tai chi, qigong, and yoga have become increasingly popular ways to reduce stress; relieve anxiety; improve balance, flexibility, and strength; and improve quality of life. It is not uncommon to see these mindful movement practices offered at fitness centers, senior centers, and studios throughout communities. Physical therapists are likely to have patients who are concurrently taking classes, have questions about modifications and the safety of certain movements, or may just be curious if the practices can improve their condition. Therapists who understand the benefits of mind-body movement can either guide their patients to other teachers or use the principles and exercises in their own therapy sessions.
The benefits of mindful movement span physical, biological, mental, emotional, and spiritual dimensions, which is why these practices fit neatly within the biopsychosocial model of healthcare. The biopsychosocial model affirms that an individual’s health is influenced by more than just the biological nature of a disease. A person’s psychological state, attitude, beliefs, environment, social and family support, and relationships with healthcare providers all contribute to the patient’s health.
Modern medicine aims to treat the whole person, which includes not only prescribing medications, but also exercise and healthy lifestyle choices. Mind-body practices treat the whole system, not just a particular body part or injury. Tai chi and qigong are recommended by major medical institutions such as the Veterans Health Administration, Harvard medical school, and the American College of Sports Medicine for their emphasis on whole-body healing and well-being. By learning simple routines that can be done at home, patients increase their self-reliance on managing their own ailments and learn to become creators of their own health.
So what are the benefits? Are all of these benefits relevant to physical therapy? Are there specific diagnoses that respond better to mindful movement? There is so much to explore and learn. Let’s dive in.
Physical Benefits of Mindful Movement
Tai Chi, qigong, and yoga exercises can be single movements or a sequence of movements linked together that focus on body awareness, intention, and deep breathing. Moving in and out of specific postures or sustaining these postures improves flexibility, builds strength, and—according to a Tai Chi and Qigong systematic review in 2010—can improve bone density. The “ready position” in tai chi and qigong is the beginning standing posture for all movements. It requires the person to feel grounded through the legs, with a slight knee bend, spine aligned over pelvis with a slight tucking of the tailbone, shoulders relaxed, and head aligned over the body. Standing meditations, common in qigong, increase the patient’s self-awareness of posture, allowing them to feel and make subtle adjustments in their alignment, while at the same time building stamina in their postural and leg muscles. Standing routines often include squatting, weight shifting, static and dynamic balance and coordination, which have been shown to reduce falls in older adults. I like to point out to patients who feel like they are too sedentary that doing a simple qigong routine in standing for just 15 minutes can be quite the workout!
Qigong also incorporates neural tension principles, which it describes as “muscle tendon changing.” Therapists will notice that certain exercises appear very similar and have the same feel and effect as neural flossing and gliding techniques.
Relaxation Response
Tai chi, qigong, and yoga practiced purely on a physical level could be thought of as “light to moderate exercise.” One is simply moving the body and loosening up the joints and muscles. What makes mindful movement meaningful and so effective is the addition of deep breathing, focus, and intention. It is the combination of slow diaphragmatic breathing and mindfulness that stimulates the vagus nerve, thereby increasing parasympathetic activity.
The parasympathetic branch of the autonomic nervous system is commonly referred to as the “rest and digest” system. Under polyvagal theory, one can be in various states of sympathetic, ventral-vagal, or dorsal-vagal parasympathetic dominance. In a sympathetic, “fight or flight” dominant state, physiological changes such as increased heart and breathing rate prepare and mobilize an individual to deal with stress and danger. In a dorsal-vagal dominant state, the body is immobilized, can experience near somnolence or “freezing,” and can occur in patients dealing with trauma or depression. Mind-body practices place the patient into a ventral-vagal dominant state, which is a relaxed and alert state optimal for healing, growth, and restoration.
Through interoceptive skills such as conscious awareness of breath and body sensations, focusing on the present moment, and moving intentionally, the patient is put into a state of flow. In the flow state, the patient feels relaxed yet observant, calm yet focused. This ideal balance of parasympathetic and sympathetic activity is most favorable for healing and resilience. Although the practices mentioned in these articles have differences in origin—for example, yoga originated in India, Tai Chi was developed as a martial art, and qigong was practiced solely for the purpose of health—all of them elicit the relaxation response. Scientific research on the benefits of mind-body practices have largely centered on the relaxation response as the key factor in improving health.
Who Benefits from Mindful Movement?
Peer reviewed scientific research supports evidence in varying degrees that mindful movement can improve heart disease, hypertension, diabetes, osteoporosis, arthritis, chronic obstructive pulmonary disease, Parkinson’s disease, multiple sclerosis, and effects from stroke. Mindful movement can reduce falls, support cancer care, improve immune function, reduce inflammation and pain, and improve depression, anxiety, mood, cognition, and quality of sleep.
I have found qigong to be highly effective in patients with fibromyalgia, dermatomyositis, and other chronic pain syndromes. These patients often comment that the movements are more gentle, and open up and release their muscle tension better than traditional physical therapy exercises. The focus on slow diaphragmatic breathing and meditation also reduces their pain. I have used slow weight shifting exercises for improving balance and control in patients with multiple sclerosis and have done simple routines that challenge arm and leg coordination and improve mental focus in elderly clients.
Mindful movement is accessible to a large portion of the population because it is gentle, low cost, does not require large spaces or any equipment, and can be done by individuals at any fitness level. Routines are highly adaptable and can be done lying down, sitting, or standing. Therapists can use mind-body principles and modify exercises for patients of any physical ability.
In the next article of this series, I will discuss several mindful movement concepts and key verbal cues to use with your patients, review a variety of qigong routines, and cover things to watch for when patients are just beginning. I find doing the routines with my patients is fun and beneficial for my own health as well and can help prevent therapist burn-out. Stay tuned!
Do you have any questions for the author or commentary about how you would use these techniques with patients or do already? Sound off in the comments!
Karen Danchalski, PT, DPT, has been practicing physical therapy for 24 years. She currently practices in an outpatient orthopedic clinic and provides outpatient services in the home setting. Karen has a special interest in mindful movement, has been a certified Stott Pilates instructor for the past 10 years, and is a member of the National Qigong Institute. She integrates Pilates and qigong exercises into traditional physical therapy treatments whenever it can benefit the patient. She is the author of several articles written for therapists on the topics of exercise for seniors, pain, and perso
[BLOG POST] Efficacy of virtual reality augmented robot-assisted gait training in chronic stroke – results from a randomized controlled single-blind trial.
Posted by Kostas Pantremenos in Gait Rehabilitation - Foot Drop, Rehabilitation robotics, Virtual reality rehabilitation on February 25, 2023
Authors: Irina Benedek, Oana Vant
3 The benefits of virtual reality-assisted neurorecovery programs
4 Study addressability and parameters evaluation
5 Results, Discussion & Study limitations
The impact of stroke
Stroke remains the leading cause of morbidity and disability worldwide, significantly impacting the quality of life. Sensor and motor difficulties in patients who suffered a cerebrovascular accident, such as loss of postural control, muscular weakness, alterations of muscle tone, and decreased cognitive skills, limit daily activities by impairing balance and walking.
Furthermore, stroke has a significant socioeconomic influence on society at a global level. In terms of media attention, patient and caregiver understanding, service advancements, and research, stroke is assuming a growing influence. Over 9 million stroke survivors make up the 4.5 million stroke fatalities annually. If they live to be 85 years old, roughly one in four males and nearly one in five women that age may anticipate having a stroke. [1–3].
Neurorehabilitation is mainly focused on the recovery processes in the acute and subacute (first 3 to 6 months) stages of stroke. Several benefits have also been observed in chronic patients (6 months post-stroke) [4]. Relearning motor skills and neuroplasticity are essential concepts when it comes to rehabilitation. Therefore, one of the aims of neurological recovery is to obtain the best results and translate the lessons into improving the patient’s everyday life.
To better undestand stroke and stroke neurorehabilitation, visit:
- Shedding light on Speech-Language Therapy: How stroke survivors can benefit from intensive rehabilitation training for chronic aphasia
- Interview with Prof. Natan Bornstein (Chair of Israeli Stroke Society)
- Interview with Prof. Marc Fisher (President of the World Stroke Organization)
Innovative technology-supported techniques for rehabilitation programs – Robotic and Virtual Reality (VR) devices
Due to their numerous benefits in managing stroke, technology-supported rehabilitation techniques like Virtual Reality (VR) and robot-assisted gait systems have recently been studied and used for this purpose. VR training has been demonstrated to:
- boost neuroplasticity
- improve movement quality
- and functional capacity in stroke patients [4].
While robot-assisted gait training (RAGT) has been proven to enhance gait and balance [5], using VR in conjunction with RAGT enables the sensory component of the nervous system to be included in rehabilitation programs. Furthermore, according to medical data, adding VR to robotic treatment helps stroke patients feel more motivated and actively participate in their recovery training [6].
The benefits of virtual reality-assisted neurorecovery programs
There are numerous VR applications in neurorehabilitation [7], as described in Figure 1 below:

Figure 1. VR Applications
Some studies reported that VR training, in addition to conventional therapy, was more effective on functional measures in patients with chronic stroke than conventional therapy alone. With a three-week course of VR combined treadmill training, Xiao et al. found an increase in activation of the primary sensorimotor cortex ipsilateral to the lesion and supplementary motor region bilaterally; an increase in gait speed was also noticed [7].
The primary goal of the study by Kayabinar and the team was to demonstrate the impact of a combined regimen of VR and RAGT on the ability of dual-task performance in chronic stroke patients. The secondary goal was to assess how VR training in conjunction with RAGT influenced chronic stroke patients’ gait, mobility, balance, fear of falling, and level of independence in daily activities [7].
Study addressability and parameters evaluation
Between February 2019 and June 2019, thirty chronic stroke patients took part in a randomized controlled single-blind trial at the inpatient rehabilitation center. They were randomly and equally assigned into two groups:
- virtual reality-augmented robot-assisted gait training (VR-RAGT) and
- control group robot-assisted gait training (RAGT) performed with RoboGait [7].
RoboGait was an exoskeleton gait robot. It included a knee and hip support that could be programmed to move a person’s lower extremities on a treadmill along a predetermined path. Overall, the technology included a treadmill, a dynamic body weight support system, and a motorized robotic orthosis that enabled patients to walk with a natural stride. The motorized gait orthosis was computer-controlled and made to facilitate joint mobility at different walking rates. In addition to RAGT, patients in the trial group engaged in two-dimensional VR gaming on the device’s 40-inch screen created by RoboGait. The patients were challenged to navigate a dense forest setting without colliding with any of the trees while attempting to gather the coins that showed on the screen, all while collecting points for the system. Moreover, both groups engaged in patient-specific neurodevelopmental therapy programs, including lower, upper, and trunk approaches based on their functional levels and requirements, in addition to the therapies [7].
The patient eligibility criteria are mentioned in Figure 2 below:
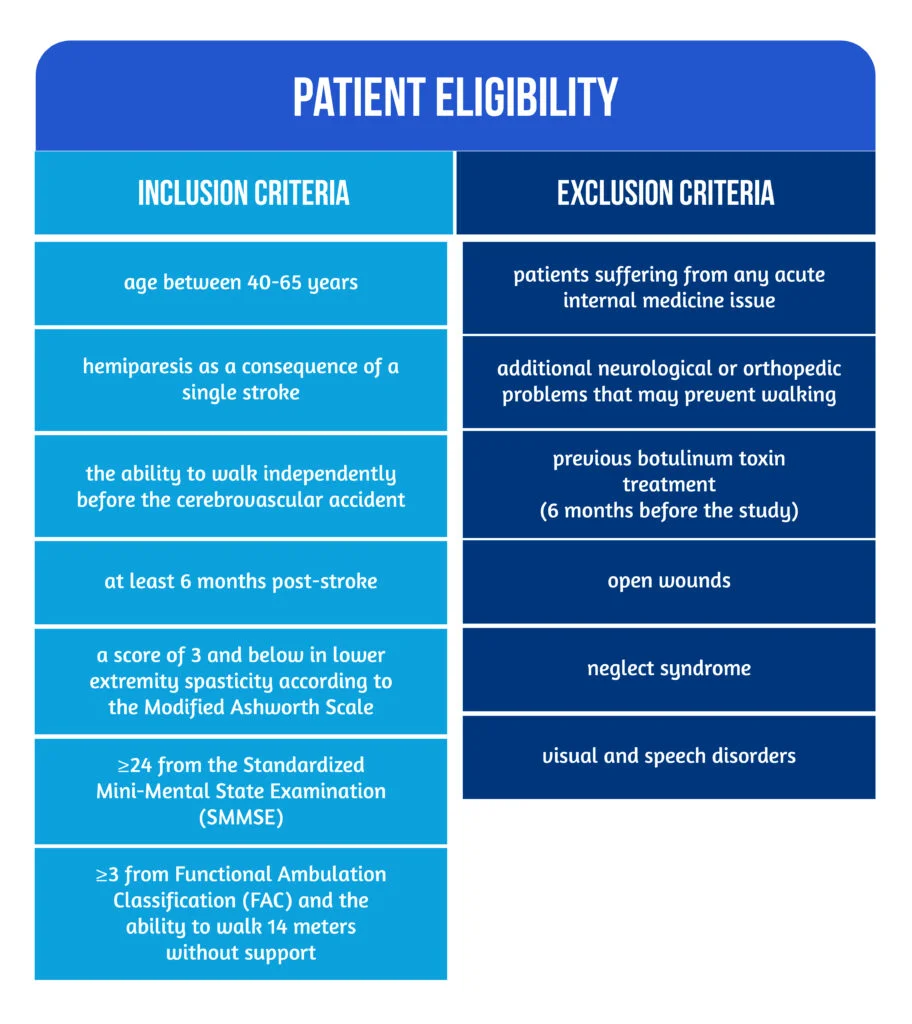
Figure 2. Patient eligibility
There were two physiotherapists in the study: one responsible for randomization and treatment, and the other responsible for assessment to avoid bias.
The functional parameters used to quantify the results were applied at baseline and after 6 weeks of treatment, as represented in Table 1.
| Parameter | Scale/ Measurement tools | Evaluation/ Details |
| Cognitive function | Mini-Mental State Examination (MMSE) | Evaluates language, attention and calculation, recollection, orientation, and registration |
| Ambulation | The Functional Ambulation Classification (FAC) | Evaluates ambulation levels including six distinct functional categories, ranging from 0 (range 0- non-functional ambulation) to 5- FAC (ambulator-independent). |
| Dual-task performance | 10-Meter Walk Test (10MWT) | Using different motor and cognitive tasks in addition to the 10-Meter Walk Test (10MWT), which represented the primary motor task. Dual-task interference is the change in performance caused by engaging in two tasks at once. The amount of dual-task interference is evaluated by using the dual-task effect (DTE) formula evaluated the amount of dual-task interference. DTE is calculated by comparing the results of the two tasks individually and jointly (DTE = dual-task performance – single-task performance). |
| Gait | The Functional Gait Assessment (FGA) | Evaluating the patients’ gait characteristics under various circumstances ( e.g., walking normally, walking at varying speeds, turning one’s head to the left and right while walking, walking on one’s heels, and climbing stairs ). |
| Mobility | Rivermead Mobility Index | Reliable for stroke patients and assesses functional mobility in various activities, from turning over in bed to jogging. |
| Balance | The Berg Balance Scale (BBS) | Evaluation of balance while performing different functional tasks |
| Fear of falling | The Fall Efficacy Scale International (FES-I) | |
| Daily life activities | Functional Independence Measure | Independence in daily tasks |
Table 1. Functional parameters and their usage [7-9]
Results, Discussion & Study limitations
In the study by Kaybinar et al., both groups underwent a non-progressive gait training program at a standard pace and with weight support. The study was a randomized, controlled, single-blind trial that examined the effects of VR-augmented RAGT on dual-task performance and functional measurements in chronic stroke patients. The findings showed that the impact of VR-augmented RAGT on the above-mentioned functional parameters was comparable to those of RAGT-only approaches [7].
After treatment, the study group’s performance on dual tasks and gait speed showed a substantial change, but there was no difference between the study and control groups. The control group, which had superior scores from baseline measures, showed no change following the therapy, according to the data. However, the research group with lower beginning performance ratings demonstrated considerable gains.
It is acknowledged that an improvement in motor skill performance in a clinic setting cannot translate to performance in real-world everyday tasks [10]. Stroke patients frequently exhibit problems in proprioception and balance, which are strongly linked to a fear of falling. As a result, the literature routinely assesses stroke patients’ fear of falling, claiming that this concern diminished during rehabilitation. Despite improvements in both research groups’ levels of patient fall fear, the improvement in the control group was around two times greater than in the study group. This is because so many things performed daily include several tasks in addition to the ones performed in therapy. Adding a second goal (getting points) to gait is an example of the multi-task element of daily walking activity. It causes the patient to get distracted, just like in virtual reality games. However, it is a predictable outcome that concentrating on a particular job (gait) will result in increased development of the fear of falling, just as it did in patients in the control group [7].
The cognitive task used in this study included the decision-making and verbal expression components of cognitive skills. This could be a limitation given that cognitive skills have complex domains such as memory, verbal memory, attention, spatial perception, expression, numerical functions, language, and executive functions. Additional cognitive skill components should be examined in future research [7].
Conclusions
Rehabilitation methods that include performing motor and cognitive multi-tasks simultaneously and training in conjunction with virtual environments linked to daily living activities may enhance the functional gains of stroke patients [7].
This study showed that chronic stroke patients’ gait speed, dual-task performance, functional gains, and independence improved with VR-augmented RAGT training, supporting the use of rehabilitation strategies in which VR is included concurrently with functional training. Only RAGT techniques improved functional assessments, degrees of independence, and fear of falling; hence it was assumed that this strategy could aid patients’ rehabilitation processes.
Further research is needed to determine whether combination therapy techniques are more effective for chronic stroke patients with higher cognitive involvement [7].
References
- Sisto SA, Forrest GF, Glendinning D. Virtual reality applications for motor rehabilitation after stroke. Top Stroke Rehabil 2002;8:11–23. DOI: 10.1310/YABD-14KA-159P-MN6F
- Wolfe CD. The impact of stroke. Br Med Bull. 2000;56(2):275-86. doi: 10.1258/0007142001903120
- Lund C, Dalgas U, Grønborg TK, Andersen H, et al. Balance and walking performance are improved after resistance and aerobic training in persons with chronic stroke. Disabil Rehabil 2018;40:2408–15. DOI: 10.1080/09638288.2017.1336646
- Iruthayarajah J, McIntyre A, Cotoi A, Macaluso S, Teasell R. The use of virtual reality for balance among individuals with chronic stroke: a systematic review and meta-analysis. Top Stroke Rehabil 2017;24:68–79. DOI: 10.1080/10749357.2016.1192361
- Cho JE, Yoo JS, Kim KE, Cho ST et al. Systematic Review of Appropriate Robotic Intervention for Gait Function in Subacute Stroke Patients. BioMed Res Int 2018;2018:4085298. DOI: 10.1155/2018/4085298
- Bergmann J, Krewer C, Bauer P, Koenig A et al. Virtual reality to augment robot-assisted gait training in non-ambulatory patients with a subacute stroke: a pilot randomized controlled trial. Eur J Phys Rehabil Med 2018;- 54:397–407. DOI: 10.23736/S1973-9087.17.04735-9
- Kayabinar B, Alemdaroğlu-Gürbüz İ, Yilmaz Ö. The effects of virtual reality augmented robot-assisted gait training on dual-task performance and functional measures in chronic stroke: a randomized controlled single-blind trial. Eur J Phys Rehabil Med. 2021;57(2):227-237. doi: 10.23736/S1973-9087.21.06441-8
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–98. DOI: 10.1016/0022-3956(75)90026-6
- Holden MK, Gill KM, Magliozzi MR, Nathan J, Piehl-Baker L. Clinical gait assessment in the neurologically impaired. Reliability and meaningfulness. Phys Ther 1984;64:35–40. DOI: 10.1093/ptj/64.1.35
- Huang HJ, Mercer VS. Dual-task methodology: applications in studies of cognitive and motor performance in adults and children. Pediatr Phys Ther 2001;13:133–40. Available at: https://pubmed.ncbi.nlm.nih.gov/17053670/
[RESOURCES] Discover the wealth of content in the World Physiotherapy congress archive
Posted by Kostas Pantremenos in resources on February 23, 2023
The World Physiotherapy congress archive now includes material from our congresses in 2017, 2019, and 2021.
The archive holds a wealth of content, including under-represented areas of research from physiotherapists in different parts of the world.
For example, when World Physiotherapy held its first congress in Africa, in Cape Town in 2017, there was a 71% increase in abstract submissions from physiotherapists in Africa and 26% of congress participants were from Africa, compared to only 3-5% in previous locations. This demonstrates the value and importance of the congress location in improving access to research and raising the profile of presenters and the work they are doing, as significant congress content does not get published in peer reviewed journals that struggle to reflect the diversity of research undertaken.
This issue was highlighted by Saurab Sharma in a session on equality, equity, diversity and inclusion during the World Physiotherapy Congress 2021 online. Saurab explored the many challenges faced by physiotherapists from different parts of the world seeking to share their research and work in journals and academic publications.
Visit the congress archive to watch the Equality, equity, diversity and inclusion session from World Physiotherapy Congress 2021 online.
You can search the archive to find recordings of sessions and presentations, videos of opening and closing sessions, ePosters, abstract presentations – and more. The archive can be searched by topic, session type, presenter, congress year and key words. Content from future congresses will be added to the archive.
————————————————————–
World Physiotherapy congress archive
World Physiotherapy Congress 2021 online
View the congress archive for 2021
Visit the congress archive for 2021
WCPT Congress 2019 in Geneva
View the congress archive for 2019
Visit the congress archive for 2019
WCPT Congress 2017 in Cape Town
View the congress archive for 2017
[WEB] Spinal stimulation can improve arm and hand movement years after a stroke
Posted by Kostas Pantremenos in Paretic Hand on February 22, 2023

Research participant Heather Rendulic prepares to grasp and move a can of tomato soup at Rehab Neural Engineering Labs at the University of Pittsburgh.
Tim Betler/UPMC and University of Pittsburgh Schools of the Health Sciences
Pulses of electricity delivered to a precise location on the spinal cord have helped two stroke patients regain control of a disabled arm and hand, a team reports in the journal Nature Medicine.
The success should give “a lot of hope” to hundreds of thousands of people in the U.S. who’ve been disabled by a stroke, says Dr. Walter Koroshetz, director of the National Institute of Neurological Disorders and Stroke, which helped fund the research.
The results will need to be replicated in a larger study, Koroshetz says, adding that it’s still unclear which stroke patients will benefit most from the treatment.
For Heather Rendulic, 33, one of the patients in the study, the treatment was life-changing.

The medical team at UPMC Presbyterian hospital prepares Rendulic for the implantation of the spinal cord stimulation electrodes.
Tim Betler/UPMC and University of Pittsburgh Schools of the Health Sciences
As a teenager, Rendulic liked to run and ride horses. Then, beginning in 2011, she had a series of strokes caused by malformed blood vessels in her brain. The last stroke was the worst.
“I woke up and I couldn’t move the whole left side of my body,” Rendulic says.
Surgeons were able to remove the cluster of blood vessels that had caused her strokes. But the damage was done.
By subscribing, you agree to NPR’s terms of use and privacy policy. NPR may share your name and email address with your NPR station. See Details. This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
“It took me almost two years to walk on my own unassisted,” says Rendulic, who wrote a book about her experiences.
Rendulic was eventually able to move her arm and hand a bit. For example, she could close her hand, but not open it. As a result, she was unable to tie her own shoes, open a jar, or chop vegetables.

University of Pittsburgh neurosurgeon Dr. Peter Gerszten (left) and assistant professor of neurosurgery Marco Capogrosso, during the implantation procedure at UPMC Presbyterian hospital.
Tim Betler/UPMC and University of Pittsburgh Schools of the Health Sciences
“You don’t realize how many things you need two hands for until you only have one good one,” she says.
So nearly a decade after her strokes, Rendulic volunteered for a study at the University of Pittsburgh.
Researchers there knew that in most people like Rendulic, the brain is still trying to send signals through the spine to the muscles that control the arm and hand. Marco Capogrosso, an assistant professor in the department of neurosurgery, says the problem is that those signals are very weak.

University of Pittsburgh kinematic occupational therapist Amy Boos (left) and Carnegie Mellon University graduate student Nikhil Verma (middle) connect muscle activation sensors on Rendulic.
Tim Betler/UPMC and University of Pittsburgh Schools of the Health Sciences
“We wanted to pick up on these weak signals and essentially turn them into functional outputs so that a person would be able to control their own hand voluntarily,” he says.
Capogrosso and a team of researchers hoped to do this by delivering pulses of electricity to nerve cells in the spine. The electricity makes these nerve cells more responsive, or excitable, which helps signals from the brain get through to the muscles they control.

(Left) A close-up of a stimulating electrode containing eight stimulation contacts. (Right) Gerszten explains the placement of stimulating electrodes while holding one in his hand.
Tim Betler/UPMC and University of Pittsburgh Schools of the Health Sciences
When the team tried this in animals, they were able to restore arm and hand function.
“If you carefully place the electrodes inside the spinal cord, you can direct this excitability toward the muscles you need,” Capogrosso says.
The team was pretty sure their approach would work in people, he says. “But we didn’t expect the amount of movement recovery that we observed.”

University of Pittsburgh graduate student Erynn Sorensen (left) observes research participant Rendulic during the isometric torque test used to measure arm strength.
Tim Betler/UPMC and University of Pittsburgh Schools of the Health Sciences
Rendulic was the first person they treated. A surgeon used a large needle to place the electrodes in her spine. “I had wires hanging out of my back,” she says.
Later, in the lab, researchers turned on the stimulation. The effect was immediate.
“I was opening my hand in ways that I haven’t in ten years and my husband and my mom were with us and we all were in tears,” Rendulic says.

Graduate students (foreground) observe a testing procedure.
Tim Betler/UPMC and University of Pittsburgh Schools of the Health Sciences
The difference is easy to see in a video made by the researchers that shows Rendulic trying to pick up a can of soup.
At first, “you can see she can’t really do anything with her hand,” says Elvira Pirondini, a research assistant professor in physical medicine and rehabilitation. “But when the stimulation is on she can reach the soup and she can grab the can and also elevate it.”
Marc Powell et al YouTube
The electrical pulses also improved something many stroke patients lose — the ability to sense the position of her arm and hand without looking at them, which comes from a sort of sixth sense known as “proprioception.”
“When the stimulation was on, it was much easier for her to understand where her arm was in space.” Pirondini says.
Rendulic gives a thumbs up while holding a fork with a piece of steak with her affected arm.
Tim Betler/UPMC and University of Pittsburgh Schools of the Health Sciences
The effects of stimulation became more dramatic during the four weeks each patient had the electrodes in their spine.
“They start by opening the hand and by the end of the four weeks they can do all sorts of things,” Capogrosso says.
Also, the effects diminished but did not disappear entirely when the stimulation was switched off. That suggests the pulses are causing changes to the circuits controlling the arm and hand, Capogrosso says, though it’s not clear how long those changes will last.
At the end of the four-week study, the electrodes were removed from both patients. But researchers say they plan to develop a system that can be implanted permanently.
Ordinarily, moving this sort of technology from the lab to widespread use takes many years. But the process is likely to move much faster in this case because the device used to stimulate the spine is already approved by the Food and Drug Administration for treating patients with chronic pain.
“There are thousands of patients implanted with this technology,” Pirondini says.
Spinal stimulation has also been used to help patients paralyzed by a spinal injury regain the ability to walk.
“I don’t see any deal breakers on the way of getting this to [stroke] patients,” Koroshetz says.
Rendulic says her experience has changed the way she views her future, and she hopes to be first in line to receive a permanently implanted stimulator.
[WEB] Helping Stroke Patients Regain Movement in Their Hands – The New York Times
Posted by Kostas Pantremenos in Paretic Hand, REHABILITATION on February 21, 2023
The results of an innovative study suggest electrical stimulation of the spinal cord could eventually help some of the many people disabled by strokes.

By Pam Belluck
- Feb. 20, 2023
Heather Rendulic was 23 when she suffered a stroke that disabled her left side. Ten years later, her left arm and hand remain so impaired that she cannot tie her shoes, type with two hands or cut her own food.
But for an extraordinary month, while participating in an innovative study, she suddenly was able to open a padlock with a key, draw a map of Italy, dip a chicken nugget in sauce and eat it with a fork — all with that left hand.
“It was like I actually had two arms, oh my gosh!” Ms. Rendulic said recently.
Researchers from the University of Pittsburgh and Carnegie Mellon University implanted electrodes along her spinal cord , delivering electrical stimulation while she tried different activities. With stimulation, her left arm had greater mobility, her fingers had more dexterity, and she could make intentional movements more quickly and fluidly.
The study, published Monday in the journal Nature Medicine, represents the first successful demonstration of spinal cord stimulation to address weakness and paralysis in the arms and hands of stroke patients.
The study was small and preliminary, involving only Ms. Rendulic and another patient. Several scientists said many questions remain about the technique’s effectiveness and applicability, but that the research suggested spinal cord stimulation could eventually help some of the many people who experience strokes.
“I think there’s enormous implications for improving quality of life,” said Dr. Lumy Sawaki-Adams, the program director in the clinical research division of the National Institute of Neurological Disorders and Stroke, who was not involved in the research. Still, she said, “we have to be cautious that we’re not offering hope to many people when I think we’re not there yet.”
Spinal cord stimulation has been used for decades to treat chronic pain. More recently, experiments delivering stimulation — either through surgically implanted electrodes or noninvasively through electrodes placed on the skin — have shown promise in helping patients with spinal cord injuries regain mobility in their legs and, in some cases, their arms and hands.
But the approach has been mostly unexplored for stroke, partly because of differences in the location and type of damage, neurological experts said.
Because strokes occur in the brain, it had been assumed that applying stimulation outside the brain would not provide “the same bang for the buck,” said Arun Jayaraman, the executive director of the technology and innovation hub at Shirley Ryan AbilityLab, a rehabilitation center in Chicago. He said the study, which he was not involved in, countered that assumption, instead suggesting that stimulating the spine, the pathway from the brain to hand and arm muscles, may help impaired limbs.
Each year, more than 12 million people worldwide and nearly 800,000 in the United States experience strokes, said Dr. Karen Furie, the vice chair of the American Stroke Association’s stroke brain health science subcommittee.
Initially, patients typically receive about six months of physical, occupational and other therapies, she said, but then progress often plateaus.
“We have virtually nothing to offer people who are years out and have longstanding disabilities,” said Dr. Furie, who is also the chair of neurology at Brown University’s Warren Alpert Medical School and was not involved in the study.
About three-quarters of stroke patients experience impairment, weakness or paralysis in their arms and hands, said Dr. Elliot Roth, an attending physician at Shirley Ryan AbilityLab’s Brain Innovation Center, who was not involved in the study. “For many people, it’s the toughest part of the stroke recovery process and tends to recover the slowest,” he said.
The patients who participated in the study had experienced different types of strokes and had varying degrees of impairment. Ms. Rendulic’s stroke was hemorrhagic, caused by bursting blood vessels. The other, more severely impaired patient, a 47-year-old woman whom researchers did not identify, experienced an ischemic stroke, which is more common and involves blocked blood vessels.
Researchers implanted strands of eight electrodes in two locations, corresponding to where neurosensory fibers from the arm and the hand enter the spinal cord.
Marco Capogrosso, an assistant professor of neurological surgery at the University of Pittsburgh, said that the approach derived from the fact that with strokes, some neural areas remain undamaged.
“So, if we can build this technology to amplify neural signals, maybe we have a chance to restore arm and hand movement,” said Dr. Capogrosso, who led the research with Elvira Pirondini, an assistant professor of physical medicine and rehabilitation at the University of Pittsburgh, and Douglas Weber, a professor of mechanical engineering at Carnegie Mellon’s Neuroscience Institute.
Five days a week for four hours each day, researchers activated the stimulation, calibrated it to determine optimal parameters for each patient and asked them to attempt various movements and tasks. Right away, the effect was noticeable.
“The very first day in the lab and the first time they turned it on, I was sitting in a chair, and they asked me to open and close my hand, and that’s something that’s really difficult for me,” Ms. Rendulic said. As her husband and mother watched, “I immediately was opening and closing my hand,” she said. “We all broke down in tears.”
Over four weeks, she was given increasingly challenging tasks, like gripping and moving a soup can. With stimulation, her left hand moved 14 small blocks over a barrier in a box, compared with six blocks without stimulation.
Typically, when Ms. Rendulic, 33, who works at home for a company’s human resources department, tries to make her left hand do something like grasp a pen, her arm feels like “it’s made of rock,” almost disconnected from her brain, she said. With stimulation “it was like my brain was able to find my left arm so much easier.”
The other patient, who was given simpler tasks because her left hand was almost completely paralyzed, improved in skills like reaching.
Researchers also tested a “sham” stimulation, activating electrodes randomly to see if patients responded to a kind of placebo effect rather than stimulation targeted specifically to their arms and hands. Both performed better with targeted stimulation.
The patients sensed the stimulation, but it didn’t cause pain, rigidity or safety problems, researchers reported.
The approved study protocol required removing the electrodes after 29 days. But one month later, the patients retained some improved abilities, surprising researchers. “We thought it was not possible” after only four weeks of stimulation, Dr. Pirondini said.
It is unclear exactly why the benefit can persist, Dr. Capogrosso said, but he hypothesized that “the same neural processes that allow these people to use this stimulation method also lead to a recovery of movement when the stimulation is off.” He added, “we’re not creating new fibers, but we’re definitely restrengthening what there is.”
Several experts noted that this pilot study was not designed to answer the most relevant question for patients: Can the improvements in laboratory tasks translate into skills that matter in daily life?
“It’s a first step among hundreds,” said Dr. Daniel Lu, a professor and vice chairman of neurosurgery at the University of California Los Angeles, who co-authored a 2016 study that showed that spinal stimulation from implanted electrodes improved hand strength and control in two spinal cord injury patients.
Dr. Lu said he believes stimulation is promising, but that its impact in the new study was difficult to evaluate because there was no comparison group and patients were not given the same regimen of intensive activities before stimulation — activities that might themselves have therapeutic benefit.
“Is it possible that you’re just exercising the patient, and the patient without the stimulation would have gotten the same effect?” he asked.
Another question neuroscientists raise is whether — or in what circumstances — it is better to surgically implant electrodes or place them on the skin, a less expensive method called transcutaneous stimulation. The new study’s authors consider surgical implantation superior because it is “much more specific,” said Dr. Weber, allowing it to “target the muscles that control the wrist and the hand.”
Others, like Chet Moritz, a professor of neurotechnology at the University of Washington, have reported improvements in spinal cord injury patients using electrodes on the skin, including benefits lasting months after stimulation ends. “It’s true we can’t tune the shoulder to this degree and the elbow to this degree and the wrist to that degree, but the nervous system seems to take care of that for us,” he said.
Several neurological experts predicted that both methods could eventually be helpful and appropriate for different patients, depending on their health and other factors. All the experts, including the study authors, said stimulation would be more effective if accompanied by rehabilitation therapies.
The study’s authors said their continuing research is evaluating patients of varying stroke severity, age and other characteristics to determine who would benefit from their approach. They have formed a company and said they envision that, as with similar technology for chronic pain, patients could adjust their stimulation via app or remote control.
If stimulation becomes regularly available to stroke patients, Ms. Rendulic would welcome it. “I did threaten to not show up to the surgery to get it removed,” she said. “I just wanted it all the time.”
While she has devised one-handed ways to do activities like driving and typing, everyday frustrations rankle, like needing her husband Mark, whom she calls “my left-hand man,” to slice steak for her.
“In the trial, I did get to cut up a steak, which was awesome,” she said. Then, fork in her left hand, she speared a piece and lifted it to her mouth — one previously impossible movement at a time.
Pam Belluck is a health and science writer whose honors include sharing a Pulitzer Prize and winning the Victor Cohn Prize for Excellence in Medical Science Reporting. She is the author of “Island Practice,” a book about an unusual doctor. @PamBelluck
[ARTICLE] Commercial device-based hand rehabilitation systems for stroke patients: State of the art and future prospects – Full Text
Posted by Kostas Pantremenos in Paretic Hand, Video Games/Exergames on February 20, 2023
Abstract
Various hand rehabilitation systems have recently been developed for stroke patients, particularly commercial devices. Articles from 10 electronic databases from 2010 to 2022 were extracted to conduct a systematic review to explore the existing commercial training systems (hardware and software) and evaluate their clinical effectiveness. This review divided the rehabilitation equipment into contact and non-contact types. Game-based training protocols were further classified into two types: immersion and non-immersion. The results of the review indicated that the majority of the devices included were effective in improving hand function. Users who underwent rehabilitation training with these devices reported improvements in their hand function. Game-based training protocols were particularly appealing as they helped reduce boredom during rehabilitation training sessions. However, the review also identified some common technical drawbacks in the devices, particularly in non-contact devices, such as their vulnerability to the effects of light. Additionally, it was found that currently, there is no commercially available game-based training protocol that specifically targets hand rehabilitation. Given the ongoing COVID-19 pandemic, there is a need to develop safer non-contact rehabilitation equipment and more engaging training protocols for community and home-based rehabilitation. Additionally, the review suggests the need for revisions or the development of new clinical scales for hand rehabilitation evaluation that consider the current scenario, where in-person interactions might be limited.
1. Introduction
Stroke, also known as cerebrovascular accident (CVA), is a cerebral blood circulation disorder leading to a sudden loss of brain function [
]. According to the Global Burden of Disease Study 2016 (GBD2016) and 2017 (GBD2017), the incidence of stroke remains high. The mortality and disability-adjusted life-years (DALYs) caused by stroke reached 6.16 million deaths and 132 million DALYs in 2017, with a significant increase from 5.5 million deaths and 116 million DALYs in 2016 [
,
]. According to the GBD2019 Stroke Collaboration [
], stroke is still the third leading cause of death and disability globally. Among the countries, China bears the highest burden of stroke, with the DALYs caused by stroke ranking first in the world [
]. According to the “China Cardiovascular Health and Disease Report”, the national stroke population in China was 13 million in 2020 [
], and the morbidity and mortality rate continues to increase year by year [
], with an anticipated increase of 35% by 2050. Studies indicate that around 85% of stroke patients worldwide have hand dysfunction, with 60% still suffering from upper limb disorders, particularly in the fingers and wrists, after treatment and discharge [
]. Hand dysfunction causes difficulties in performing the most basic activities of daily living (ADLs), such as tying shoelaces, twisting towels, and picking up and manipulating objects, greatly impacting the individual’s quality of life [
].
The rehabilitation of fine hand activity is important for recovering upper limb function. Traditional treatment requires patients to receive regular and continuous physical therapies in professional rehabilitation institutions or hospitals [
]. The therapist assesses the patient’s conditions through observation, communication, questionnaires, and functional tests [
]. Current clinical scales used for upper limb assessment include the Fugl-Meyer Assessment (FMA) [
], Wolf Motor Function Test (WMFT) [
], Box and Block Test (BBT) [
], and Action Research Arm Test (ARAT) [
], and they have been validated through the existing reports. However, these scales are focused on upper limb movements rather than hand movements, and they are time-consuming, subjective, and inflexible [
]. For example, the Jebsen-Taylor Hand Function Test (JTHFT) [
] is a specific test for hand function, but it is not frequently updated, which raises doubts about its effectiveness. Conventional rehabilitation training is dependent on the therapist’s experience and can be labor-intensive and time-consuming; this can lead to a heavy work burden for physical therapists, which can affect the effectiveness of treatment [
]. Therefore, with the development of robotic and smart technologies, advanced rehabilitation devices and innovative approaches are emerging as alternative solutions to rehabilitation treatment.
Stroke rehabilitation is typically classified into three stages based on the level of the patient’s clinical manifestations: acute, recovery, and tertiary [
]. It can also be classified as mild, moderate, and severe based on the degree of injury. In the acute stage, patients are mostly unable to perform basic life skills independently and require professional rehabilitation staff to arrange occupational therapy (OT) [
]. Rehabilitation in this stage is primarily carried out in rehabilitation centers and may include the use of rehabilitation robots (e.g., powered exoskeleton or end-effect based rehabilitation robotics) and physiological signal-based equipment (e.g., brain-computer interface (BCI) and functional electrical stimulation (FES)) [
].
During the recovery and tertiary stages, the conditions of mild or moderate stroke patients are relatively stable, and they are eager to return home [
]. Rehabilitation in the community and at home is a more practical setting for post-rehabilitation, but providing patients with complicated and costly equipment in these settings is risky and impractical. This situation calls for the development of community and home-use rehabilitation devices or technology that are safe, easy to use and store, and low cost. Patients prefer non-contact devices as they are easier to use and highly adaptable. Additionally, non-contact devices do not need to be sterilized regularly, making them particularly practical during the COVID-19 pandemic [
].
Hand rehabilitation after a stroke is a long process, and motivation is crucial for the patient’s outcome [
]. The treatment outcome depends not only on the physical therapist’s rehabilitation process but also on the patient’s motivation for the training protocols [
]. To make rehabilitation more appealing, game-based training protocols are incorporated into the training system, such as 2D games, 3D games [
], virtual reality (VR) games [
], augmented reality (AR) games [
], etc. The use of games in training improves the enjoyment of rehabilitation, allowing patients to perform voluntary or active exercises and providing feedback and encouragement repeatedly [
]. It is worth noting that the use of virtual reality in game-based training protocols meets both the physiological and psychological needs of patients. VR creates a dynamic and motivating environment by merging touch, hearing, and vision, making patients more likely to participate in clinical or home training [
]. However, developing an effective rehabilitation game is challenging. Games that are too challenging for patients can be frustrating, and games that are too easy can quickly lose the patient’s interest [
].
Some patients may indeed experience failure and lack confidence when first playing training games for rehabilitation, which lead to decreased motivation to continue playing [
]. It is particularly true for stroke patients recovering from hand disorders, as there are currently limited options for games that are specifically designed to target these conditions. Some studies have shown positive outcomes from using games in rehabilitation [
], but more research is needed to develop games tailored to the specific needs of stroke patients with hand disorders.
Many hand rehabilitation devices and game-based training protocols have been reported, but most of them are self-made and have not been clinically tested. Meanwhile, few reviews have focused on the effectiveness of the existing commercial training systems with various game-based training protocols for hand rehabilitation. Therefore, this systematic review focused on evaluating the existing commercial training systems and the various game-based protocols used in hand rehabilitation. Furthermore, the review analyzed the hardware, game-based training protocols, and clinical outcomes. This review also provided future perspectives to guide the development of hand rehabilitation in the community and home settings during and after the COVID-19 pandemic.[…]
[ARTICLE] Shoulder Joint Hybrid Assistive Limb Treatment for Chronic Stroke Patients with Upper Limb Dysfunction – Full Text
Posted by Kostas Pantremenos in Paretic Hand on February 12, 2023
Abstract
Upper extremity dysfunction after stroke affects quality of life. Focusing on the shoulder joint, we investigated the safety and effectiveness of rehabilitation using a shoulder joint hybrid assistive limb (HAL). Eight patients with chronic stroke and upper extremity functional disability were enrolled and used a shoulder joint HAL, which assisted shoulder movement based on the user’s intention, through myoelectric activation of the shoulder flexor. Ten training sessions of 30–40 min each were performed to assist voluntary movement of upper limb elevation on the affected side through triggering the deltoid muscle. All patients completed the interventions without shoulder pain. Surface electromyography evaluation indicated post-intervention improvement in coordinated movement of the affected upper extremity. Significant improvements in voluntary and passive shoulder joint range of motion were obtained after the intervention, suggesting improvement in shoulder muscle strength. A significant decrease in the modified Ashworth scale and improvements in functional scores in the upper limb were also observed. Along with safe use for our study patients, the shoulder HAL provided appropriate motor learning benefits. Improvements in shoulder joint function and whole upper limb function were observed, suggesting that HAL could be an optimal treatment method.
1. Introduction
Upper extremity dysfunction due to stroke significantly affects activities of daily living, influencing quality of life [1]. It has been reported that approximately 50% of patients with stroke continue to experience upper limb dysfunction six months after stroke onset, and approximately 60% of those with severe or complete paralysis are unable to perform any movement with their affected limbs [2,3,4]. Recent advances in imaging examinations, such as functional magnetic resonance imaging and near-infrared spectroscopy, have shown that brain plasticity or reorganization can be expected after stroke. Recently, robotic rehabilitation has emerged as a training method to improve patients’ limb dysfunction post-stroke [5,6]. The hybrid assistive limb (HAL) is an exoskeletal robot that controls and assists movements based on bioelectrical activity generated through voluntary movements. By means of generating feedback to the central nervous system, it has been hypothesized that this device stimulates functional recovery through inducing plasticity in the impaired central nervous system [7]. There are four types of HAL, namely, lower limb, single joint (for elbow and knee joints), and lumbar types. One study that used a single joint HAL for the elbows reported improvements in upper limb motor function in patients with stroke [8]. Focusing on shoulder dysfunction after stroke, we previously conducted training using a shoulder joint HAL developed by our research group in patients with stroke. Furthermore, we published a case report showing that this training could be performed safely, while improving shoulder joint function and coordinated movements of the upper limb on the affected side [9]. Rehabilitation therapy focusing on the shoulder joint is extremely important, as improving shoulder joint function not only improves activities of daily living, such as changing clothes, but also ameliorates distal control of the upper extremity and prevents shoulder pain [10,11,12]. In this study, we aimed to determine the utility of shoulder joint HAL training applied in eight patients with chronic stroke and moderate-to-severe upper limb dysfunction.
2. Materials and Methods
2.1. Patients
Eight patients (six males, two females) were enrolled in this study. Patients’ clinical data are shown in Table 1. The mean patient age (± standard deviation) was 68.4 ± 8.38 (range, 53–84) years. The mean time from stroke onset was 5.86 ± 6.40 (range, 0.93–19.7) years. All patients showed moderate-to-severe hemiplegia with a shoulder flexion manual muscle test (MMT) score of ≤2. Their grip power was <50% on the affected side compared with the unaffected upper limb and three patients were unable to complete grip dynamometer measurements. In Patient 8, bilateral grip power measurements could not be measured as the unaffected upper limb had been amputated at the hand level due to trauma in childhood. This study was conducted in accordance with the Declaration of Helsinki, with approval from the Ethics Committee of the Tsukuba University Faculty of Medicine (approval no.: TCRB18-38). All patients provided written informed consent for participation and publication, including the use of any accompanying images.
Table 1. Patient characteristics.

2.2. HAL Intervention
We set up the single-joint HAL in accordance with previous studies [5,6]. In brief, the proximal section of the HAL was fixed to a tripod using an attachment, and the distal section was fitted to the patient’s upper arm with a belt for the elbow joint, which was attached to the HAL (Figure 1a). The elbow was extended to its full range of motion (ROM); the forearm was placed in a slightly externally rotated position to prevent external rotation of the humerus and was immobilized near the wrist joint using a splint and bandage (Figure 1b). Flexion electrodes were placed on the skin of the anterior deltoid fibers and triggered for upper limb elevation. Instead of using the electrodes as triggers, relaxation of the shoulder flexor muscle and gravity functioned to trigger extension. The ground was placed on the bone touching the site where the bone was palpable, without interfering with the surface electromyography device.
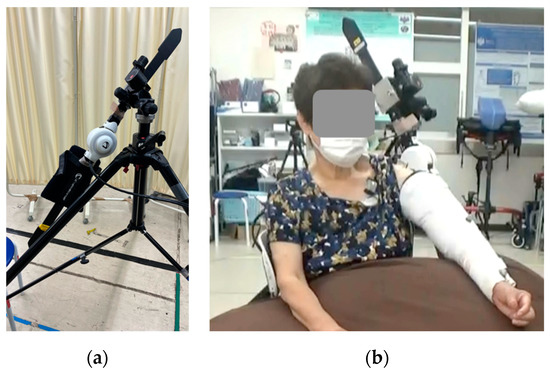
Figure 1. Images of the shoulder joint HAL device. (a) Single-joint HAL fixed to a tripod with attachments, (b) shoulder joint HAL fixed with splints and an elastic bandage. HAL, hybrid assistive limb.
The upper limb raising angle during training was measured prior to fitting the HAL, and the shoulder joint ROM was initiated at approximately 20° less than the ROM at the shoulder joint and then gradually increased while observing the training condition. Two methods were used to adjust the actual angle: (i) adjustment of the HAL, and (ii) adjustment using a tripod attachment. The HAL angle could be adjusted from 0° to 120°, and was used for adjustment. When the HAL assist angle of 120° was considered to be insufficient, a further increase in the angle of elevation was obtained through changing the tilt of the HAL itself using a tripod attachment.
All eight patients who participated in the study underwent a total of 10 HAL training sessions, with each session lasting 30–40 min, with at least one week between each intervention. The actual training time for upper extremity raising was approximately 20–30 min, including breaks, after approximately 5–20 min for electrode preparation and HAL placement and removal.
During training, a therapist stabilized the medial side of the patients’ forearms to avoid excessive internal rotation or flexion of the upper limbs during the raising of the upper limbs. The direction of upper limb elevation was evaluated while observing the raising of the scapular plane, which needs to be considered to prevent excessive interference between the humerus and scapula (Figure 2). The pace of each exercise was set so that patients could fully extend their arms one at a time to avoid vigorous raising and then repeat raising the upper arm. […]
[ARTICLE] Upper Limb Function Recovery by Combined Repetitive Transcranial Magnetic Stimulation and Occupational Therapy in Patients with Chronic Stroke According to Paralysis Severity – Full Text
Posted by Kostas Pantremenos in Paretic Hand, tDCS/rTMS on February 12, 2023
Abstract
Repetitive transcranial magnetic stimulation (rTMS) with intensive occupational therapy improves upper limb motor paralysis and activities of daily living after stroke; however, the degree of improvement according to paralysis severity remains unverified. Target activities of daily living using upper limb functions can be established by predicting the amount of change after treatment for each paralysis severity level to further aid practice planning. We estimated post-treatment score changes for each severity level of motor paralysis (no, poor, limited, notable, and full), stratified according to Action Research Arm Test (ARAT) scores before combined rTMS and intensive occupational therapy. Motor paralysis severity was the fixed factor for the analysis of covariance; the delta (post-pre) of the scores was the dependent variable. Ordinal logistic regression analysis was used to compare changes in ARAT subscores according to paralysis severity before treatment. We implemented a longitudinal, prospective, interventional, uncontrolled, and multicenter cohort design and analyzed a dataset of 907 patients with stroke hemiplegia. The largest treatment-related changes were observed in the Limited recovery group for upper limb motor paralysis and the Full recovery group for quality-of-life activities using the paralyzed upper limb. These results will help predict treatment effects and determine exercises and goal movements for occupational therapy after rTMS.
1. Introduction
Motor paralysis after stroke limits patients’ activities of daily living (ADL) and reduces their quality of life [1,2]. Recently, noninvasive brain stimulation therapy has been developed to improve patients’ motor paralysis and ADL, and its effectiveness has been demonstrated [3,4]. The treatment of upper limb motor paralysis involves modulation of interhemispheric inhibition and induction of neuroplasticity in the cerebrum. A novel intervention using repetitive transcranial magnetic stimulation (rTMS) in combination with intensive occupational therapy (NEURO) has recently been developed [5]. In patients with stroke hemiplegia, high-frequency rTMS has been applied to the hemisphere ipsilateral to the paralysis to increase excitability [6], and low-frequency rTMS has been applied to the contralateral hemisphere to decrease interhemispheric inhibitory connections [7,8] with the damaged cortex [9]; thus, both high-frequency rTMS and low-frequency rTMS have been applied [10]. Repetitive currents are induced in the brain cortex to produce long-term changes in cortical excitability. In acute patients, high-frequency (10 Hz) rTMS applied to the impaired motor cortex activates it, improving paralysis [11,12]. In occupational therapy after rTMS, the patients in whom the activation of the interhemispheric inhibitory motor cortex has been adjusted are prescribed repetitive joint movements. The aim is to promote use-dependent plasticity in the brain and to subsequently restore motor paralysis and improve ADL [13]. NEURO is an effective treatment for improving upper limb dysfunction and impairments in ADL in chronic stroke patients 6 months after stroke onset. Its therapeutic effect has been shown to be unaffected by stroke type (cerebral hemorrhage or cerebral infarction) [14].
The goal of NEURO is to improve the quality of movement of the patient’s paralyzed upper limb by allowing it to be used in ADL. Since the effectiveness of NEURO depends on the severity of motor paralysis, therapists determine the exercises and target movements based on the patient’s pre-treatment upper limb function assessment score. The Fugl–Meyer Assessment of the Upper Extremity (FMAUE) and the Action Research Arm Test (ARAT) are used to assess upper limb motor function outcomes in NEURO [15]. These evaluation methods have been shown to have high accuracy and clinical usefulness. A previous study has been conducted to estimate post-treatment scores from the pre-NEURO FMAUE score [16]. The ARAT is a functional upper limb assessment tool used in patients with post-stroke hemiplegia and is characterized by its ability to reflect the patient’s activity [17]. Since the ARAT consists of object manipulation and reaching tasks, the occupational therapist (OT) plans exercises by estimating the ADLs in which the patient can use their hands based on the obtained assessment results. As the ARAT score correlates with the Motor Activity Log, which investigates the use of the paralyzed limb in ADLs, OTs helping patients improve their activity limitations can use it as a reference value for exercises and goal-setting [18,19]. Therefore, it can be inferred that predicting treatment effects with ARAT is more advantageous than using FMAUE in setting treatment goals and planning effective ADL exercises for patients. If ARAT scores are found to improve with NEURO, it will be easier for OTs to pre-determine the content of ADL exercises and develop achievable ADL goals.
Patients with mild-to-moderate motor paralysis with FMAUE scores ≥43 have higher interhemispheric inhibition from the healthy hemisphere to the affected hemisphere. It is predicted that the therapeutic effect of upper limb practice in the presence of rTMS-induced changes in synaptic transmission efficiency is dependent on motor paralysis severity [20]. If the post-treatment effects according to motor paralysis severity can be predicted using pre-treatment ARAT scores, the target movements for patients could be set with high accuracy. Recently, a treatment method using a brain-computer interface (BCI) was developed for the rehabilitation of stroke patients, and its effectiveness has been reported [21,22]. Even for new intervention methods, it is better to formulate exercises adapted to the severity of paralysis and recovery. Therefore, the results obtained in this study can be used as data to plan the most appropriate practice for patients in terms of future new intervention methods. As a result, this study aimed to estimate the amount of change in ARAT scores for each level of motor paralysis severity, classified according to the ARAT score before NEURO. […]
[BLOG POST] Robot-assisted neurocognitive rehabilitation of the hand
Posted by Kostas Pantremenos in Cognitive Rehabilitation, Paretic Hand, Rehabilitation robotics on February 12, 2023
Authors: Irina Benedek, Oana Vanta
1 Innovations in neurorehabilitation of the upper limbs
2 A multimodal approach to robot-assisted neurocognitive rehabilitation of the hand
4 The roles of robotic devices in improving upper extremity function
5 Results, discussions, and future perspectives
6 Conclusion on robot-assisted neurocognitive rehabilitation of the hand
Innovations in neurorehabilitation of the upper limbs
Is robot-assisted neurocognitive rehabilitation of the hand effective? Stroke is the leading cause of mortality and disability worldwide, negatively impacting the overall quality of life of patients. Most stroke survivors suffer from different types of motor impairments, despite the progressions in the field. Since neurorehabilitation of the upper limbs remains challenging, robotic-assisted therapies have been studied and developed in recent years. These methods were established as safe and practical therapies were used additionally to neurorehabilitation programs [1]. More precisely, in the last two decades, robotic devices that focused on training the proximal upper extremity were assessed, showing results similar to dose-matched conventional therapies [2].
For more information on upper limb neurorehabilitation, visit:
- The feasibility of repetitive sensory stimulation in the rehabilitation of the upper limb after a stroke (the PULSE-I study)
- Wearable elbow robot in rehabilitation after a stroke
- Recovery of precise hand movements after stroke
A multimodal approach to robot-assisted neurocognitive rehabilitation of the hand
Distal arm sensorimotor function is essential for improving the quality of life after a stroke. Nevertheless, the distal arm function is crucial for the ability to perform daily activities and is usually severely affected after a cerebrovascular accident when there is a low probability of regaining its full function [3]. However, scientific data demonstrates the possibility of recovery with intensive motor training provided by robotic-assisted devices [4, 5]. Until now, the focus has been on movement practice without implementing a therapeutic paradigm adapted to the capabilities of the technology in question.
In the study by Ranzani et al. [6] from 2020, the goal was to investigate sensorimotor robotic-assisted rehabilitation of hand function and cognition training in patients with subacute stroke. The researchers based their comprehensive approach on the fact that cognition is essential for appropriate interactions between body and environment, such as [6]:
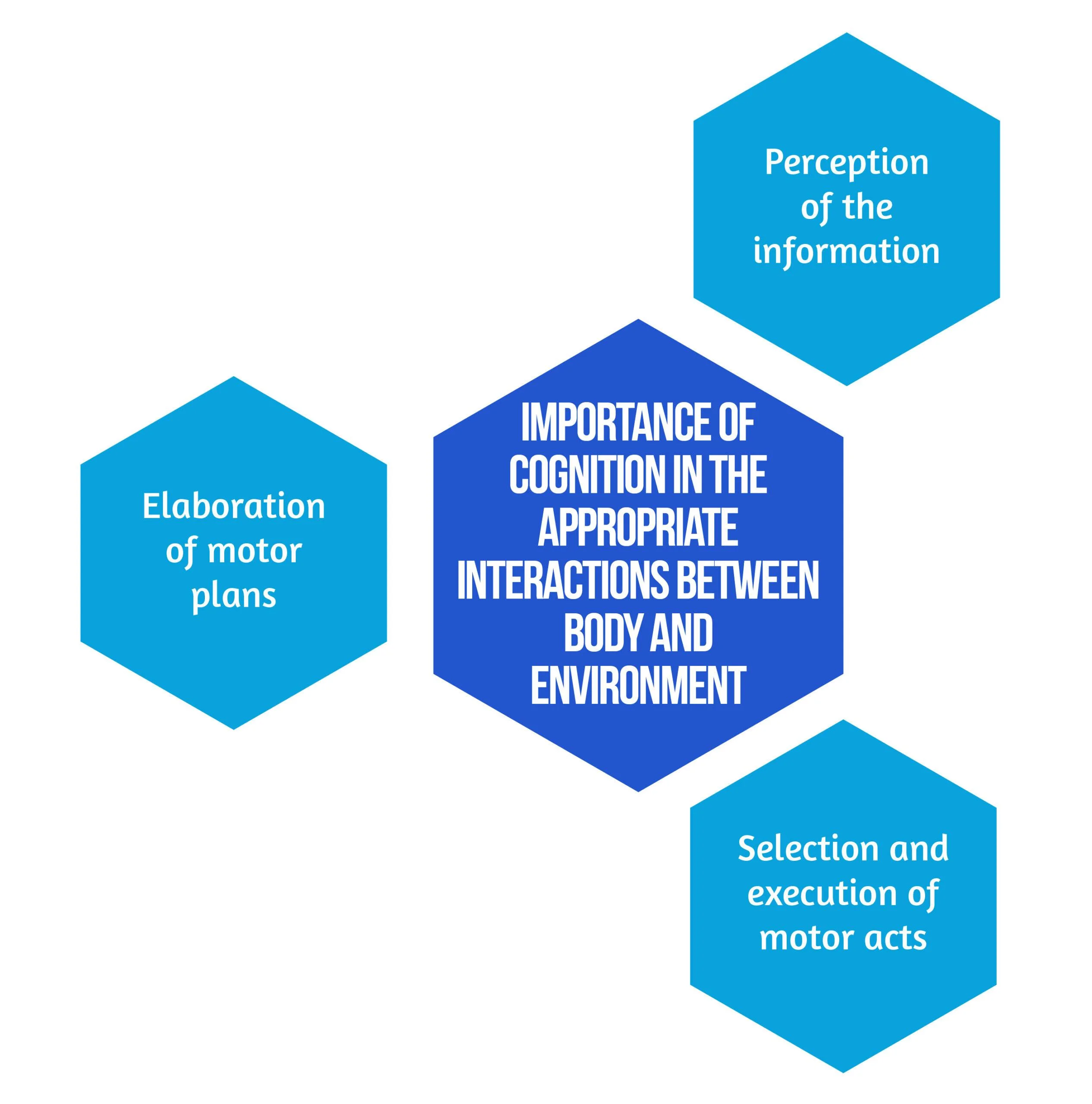
Figure 1. Importance of Cognition
Secondary objectives derived from the hypothesis are that neurocognitive robotic-assisted hand recovery would also improve motor, sensory, and cognitive functions in this category of patients. This method is particularly relevant for hand rehabilitation due to the importance of the cognitive processing of sensory information. Furthermore, combining multimodal inputs requires the involvement of associative cortices that are essential for learning and, consequently, neuronal plasticity and recovery [7]. Even though few studies have compared neurocognitive therapies to other rehabilitation treatments [8, 9], preliminary evidence suggested promising results in the following:
- Enhancing upper limb function
- Improving the ability to conduct daily tasks
- The overall quality of life [8,9].
Inspired by the neurocognitive approach, the authors [6] implemented this concept on a robotic-assisted device focused on exercises including grasping and pronation-supination. Virtual objects were produced by the robot, both visually and haptically, simulating the tangible materials used in traditional recovery [6].
Study design
The study conducted by Ranzani et al. [6] was a randomized control trial that took place in Switzerland. Patients were randomly assigned into two groups: the robot-assisted group (RG), which benefited from neurocognitive therapy with “ReHapticKnob” (Fig. 2), and the control group (CG), which received dose-matched classical neurocognitive therapy. With the main focus on hand function, all participants had three neurocognitive therapy sessions per day over four weeks. Each session lasted 45 minutes, and in the RG, one session was replaced by robot-assisted therapy. The subjects were under the careful supervision of a specialized therapist who adapted the intensity and difficulty level of the exercises according to every patient. The type and number of exercises performed in the RG group were also assessed to match the therapy type and dose performed in conventional recovery programs [6].

Figure 2. Picture of a subject with stroke, using the rehabilitation robot ( available from [6])Between April 2013 and March 2017, 33 patients who suffered a subacute stroke were evaluated and included in the study. Seventeen subjects were randomized in the RG, while 16 were allocated to the CG. 27 patients benefited from the allocated therapy and completed the T1 assessment, while 23 completed the entire protocol. Six patients withdrew before the T1 evaluation due to medical reasons or lack of motivation. No adverse events related to the interventions were noted [6]. The eligibility criteria are described in Figure 3 below:
Figure 3. Patient Eligibility
The roles of robotic devices in improving upper extremity function
As mentioned above, the neurocognitive method proposed in this study implied both sensorimotor and cognitive components, which are equally important during the performance of complicated tasks and daily activities. Patients were instructed to explore different objects, such as sponges, sticks, and strings, to discern their properties by using haptic and postural senses. A robotic device is an ideal therapeutic instrument for performing such tasks because it can render a complex range of stimuli in a repeatable, well-controlled manner [10].
The neurorehabilitation program provided in the RG group consisted of seven types of exercises [6], as showcased in Figure 4 below:
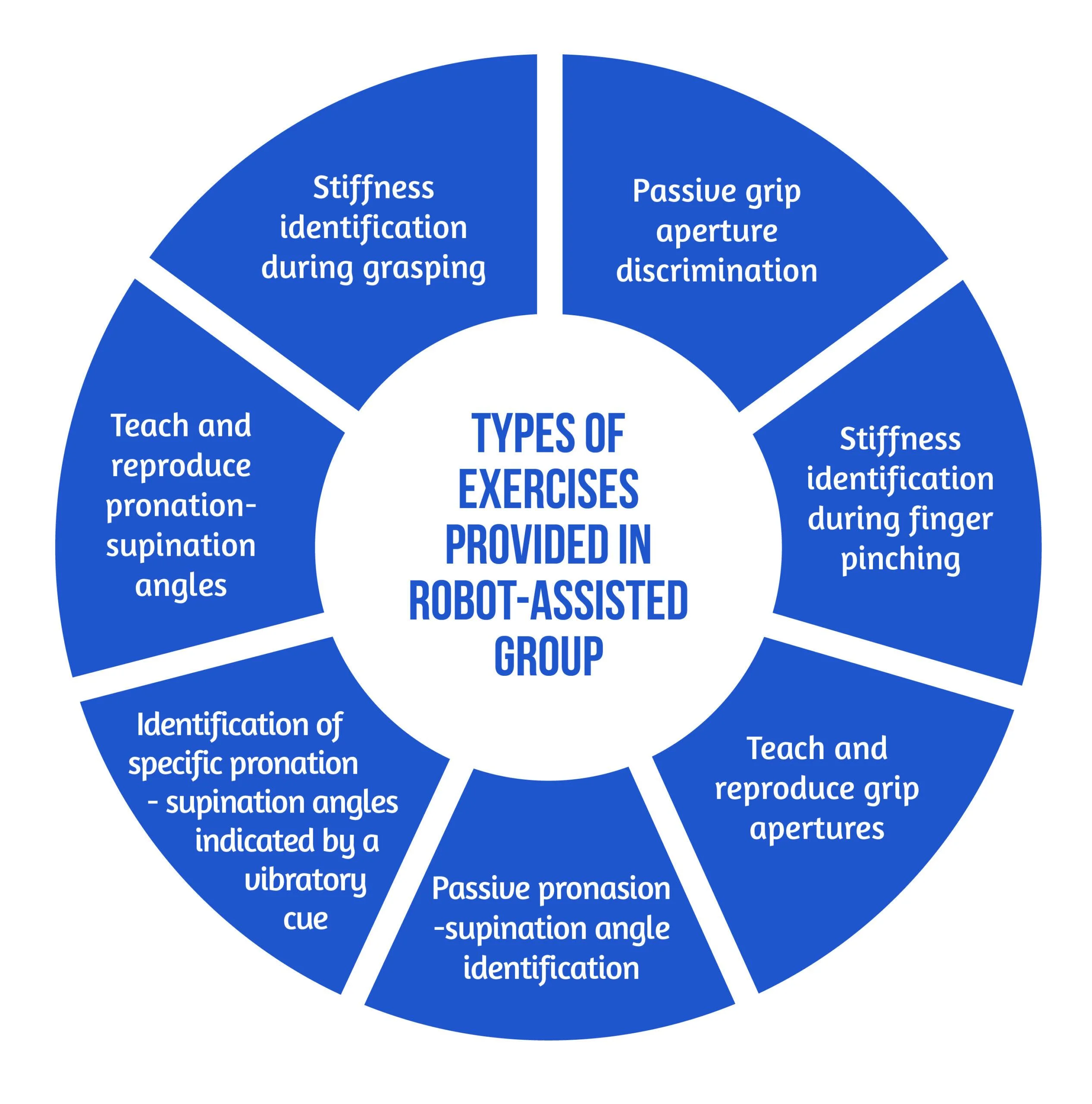
Figure 4. Types of Exercises
The motor aspects of the intervention included symmetric thumb and finger flexion/extension and forearm pronation/supination, executed separately or combined. The sensory aspects included encoding the following types of somatosensory signals without visual information: sponge/spring stiffness, object shape and size, arm positioning, and vibratory cues. The cognitive element of the training, executed passively (with guidance from the robot or therapist) or actively (by the subject), required the elaboration/recognition of perceptual information (object length, stiffness) and encoding/decoding of this information in working memory for comparison purposes of more than one item, along with planning and executing the specific motor plans [6].
The study’s primary outcome was evaluating the changes in upper limb extremity motor impairment from baseline to the end of treatment (T0-T1). The parameter used to quantify the results was the Fugl-Meyer Assessment of the Upper Limb Extremity scale (FMA-UE). Different motor, sensory and cognitive scales were also used for the secondary outcomes. The average number of task repetitions and therapy intensity were assessed to compare the two study groups in terms of dose matching. For the acceptance of the neurocognitive robotic-assisted program, a 4-item questionnaire was developed. The two study groups were evaluated and compared for all outcome measures after the intervention (T-T0) and at the regular follow-ups (T2-T0 and T3-T0) [6].
Results, discussions, and future perspectives
According to the equivalence analysis, the change in FMA-UE in the robotic-assisted group may be regarded as non-inferior to the control group. Both groups showed improvements in all secondary clinical scores after 4 weeks of therapy (T1). In addition to motor impairments, sensory and cognitive deficits were also improved in both groups after 4 weeks of treatment (T1) [6]. However, by the end of the study, subjects randomized in the RG group tended to show better results. At T2 and T3, the changes in FMA-UE were also sustained. When comparing changes in clinical measures over time, the two groups did not demonstrate any significant between-group differences [6].
According to the supervising therapist, the RG did an average of 71.49 task repetitions during a treatment session, compared to 73.47 in the Control Group. There was no statistically significant difference in therapy intensity between the two groups when comparing robot-assisted and traditional therapy sessions [6].
Regarding neurocognitive therapy sessions, the average daily quantity of occupational therapy and/or lower limb kinesiotherapy did not differ statistically between the groups.
The acceptance of the neurocognitive robot-assisted therapy was evaluated by using a self-reported questionnaire answered by 12 patients [6], as showcased in Figure 5:
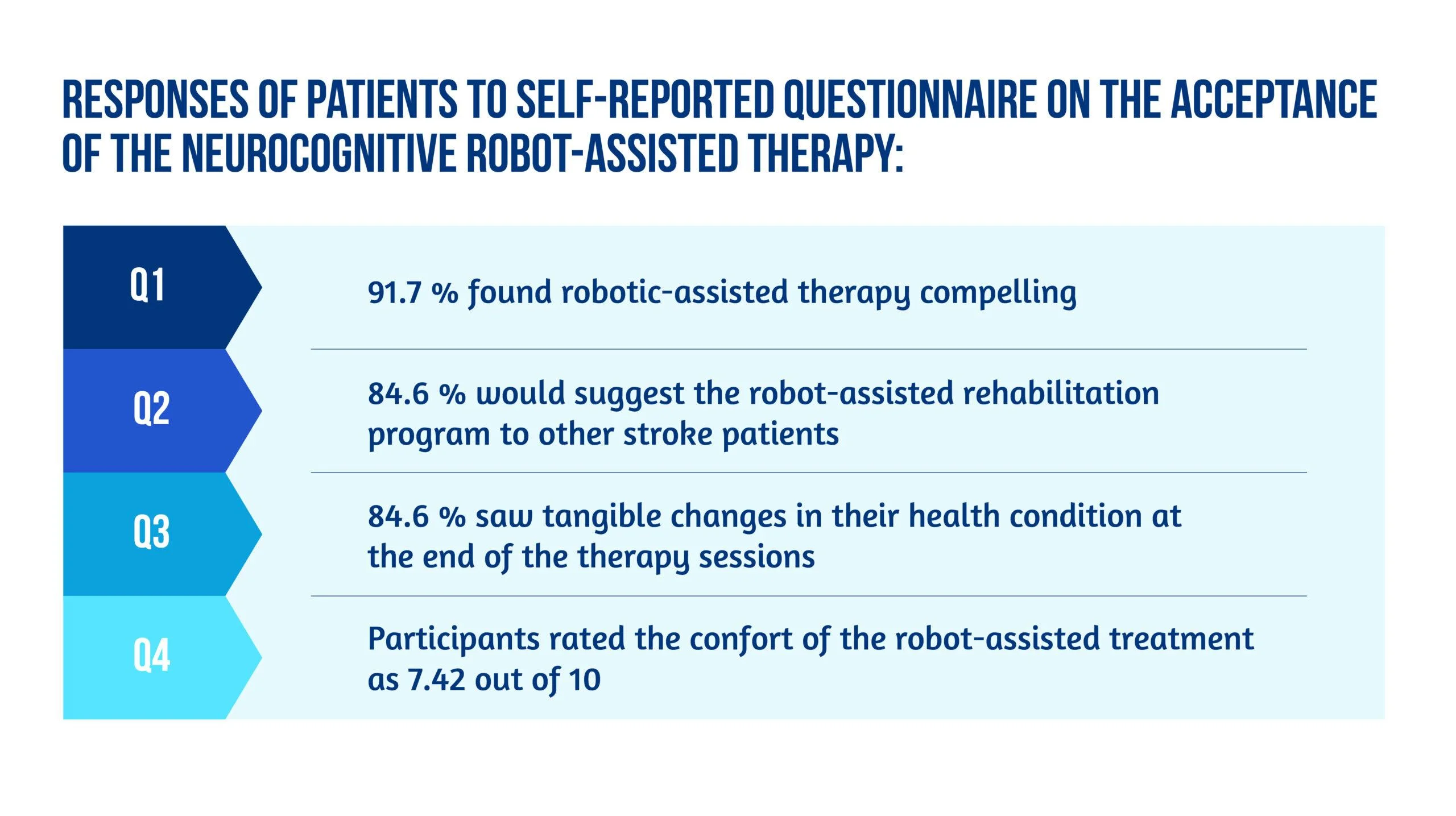
Figure 5. Patient responses
Unlike other robot-assisted rehabilitation studies that focus primarily on locomotor training, this method fully utilized the robot’s haptic rendering capabilities and suggested a therapy regimen tailored to this potential [6].
Ranzani and the team were able to demonstrate that this strategy was well-liked and recommended by the majority of patients. It could also be included in the patient’s daily routines in the subacute stage of stroke recovery. Although the questionnaire pointed out slight pain in finger fixation and difficulty levels were sometimes evaluated as excessively high in three out of seven exercises [6], most subjects considered the program encouraging and pleasant, and they noticed tangible benefits to their health after completing it.
The findings of the equivalency test assessing the evolution in the FMA-UE show that motor recovery in the RG was not inferior to the CG for the specific intervention. Usually, little to no difference between traditional and robotic-assisted therapies should be expected since the dosage and therapeutic movements are meant to be identical between the study groups. Furthermore, the idea that a traditional treatment session might be replaced without compromising the overall rehab programs opens up new possibilities for robot-assisted therapy development [6].
Conclusion on robot-assisted neurocognitive rehabilitation of the hand
This research reported the findings of a randomized controlled trial that aimed to compare robotic-assisted recovery to conventional therapy for patients with a subacute stroke and impairment of hand function. Although the study included a limited number of participants, the results showed that robot-assisted treatments might be successfully incorporated into clinical neurorehabilitation routines [6]. In other words, this trial is also an indicator of how low data can become significant data. Compared to conventional dose-matched neurocognitive programs, the results demonstrated that this innovative approach was at least as practical as the conventional one.
Early exposure of stroke patients to using such patient-tailored robot-assisted therapy programs opens the door to using such technology in the clinic with little therapist supervision or at home after hospital release to help enhance the dose of hand therapy for stroke patients [6].
Bibliography
- Veerbeek JM, Langbroek-Amersfoort AC, van Wegen EE, Meskers CG, Kwakkel G. Effects of robot-assisted therapy for the upper limb after stroke: a systematic review and meta-analysis. Neurorehabil Neural Repair. 2017; 31(2):107–21. doi: 10.1177/1545968316666957
- Maciejasz P, Eschweiler J, Gerlach-Hahn K, Jansen-Troy A, Leonhardt S. A survey on robotic devices for upper limb rehabilitation. J Neuroeng Rehabil. 2014;11(1):3. DOI: 10.1186/1743-0003-11-3
- Fischer HC, Stubblefield K, Kline T, Luo X, Kenyon RV, Kamper DG. Hand rehabilitation following stroke: a pilot study of assisted finger extension training in a virtual environment. Top Stroke Rehabil. 2007;14(1):1–12. DOI: 10.1310/tsr1401-1
- Lambercy O, Dovat L, Yun H, Wee SK et al. Effects of a robot-assisted training of grasp and pronation/supination in chronic stroke: a pilot study. J Neuroeng Rehabil. 2011;8(1):63. doi: 10.1186/1743-0003-8-63.
- Hsieh YW, Lin KC, Wu CY, Shih TY, Li MW, Chen CL. Comparison of proximal versus distal upper-limb robotic rehabilitation on motor performance after stroke: a cluster controlled trial. Sci Rep. 2018;8(1):2091. doi: 10.1038/s41598-018-20330-3
- Ranzani R, Lambercy O, Metzger JC, Califfi A et al. Neurocognitive robot-assisted rehabilitation of hand function: a randomized control trial on motor recovery in subacute stroke. J Neuroeng Rehabil. 2020 Aug 24;17(1):115. doi: 10.1186/s12984-020-00746-7.
- Van de Winckel A, Wenderoth N, De Weerdt W, Sunaert S et al. Frontoparietal involvement in passively guided shape and length discrimination: a comparison between subcortical stroke patients and healthy controls. Exp Brain Res Springer. 2012;220(2):179–89. doi: 10.1007/s00221-012-3128-2
- Sallés L, Martín-Casas P, Gironès X, Durà MJ et al. A neurocognitive approach for recovering upper extremity movement following subacute stroke: a randomized controlled pilot study. J Phys Ther Sci. 2017;29(4):665–72. doi: 10.1589/jpts.29.665
- Lee S, Bae S, Jeon D, Kim KY. The effects of cognitive exercise therapy on chronic stroke patients’ upper limb functions, activities of daily living and quality of life. J Phys Ther Sci. 2015;27(9):2787–91. doi: 10.1589/jpts.27.2787
- Metzger J-C, Lambercy O, Califfi A, Dinacci D et al. Assessment-driven selection and adaptation of exercise difficulty in robotassisted therapy: a pilot study with a hand rehabilitation robot. J Neuroeng Rehabil. 2014;11(1):154. Available at: https://jneuroengrehab.biomedcentral.com/articles/10.1186/1743-0003-11-154

