Posts Tagged NMES
[Abstract + References] Non-Invasive Brain Stimulation Combined with Neuromuscular Electrical Stimulation for Upper Limb Rehabilitation in Stroke Survivors: A Systematic Review
Posted by Kostas Pantremenos in Paretic Hand, REHABILITATION on January 18, 2024
Abstract
Purpose of Review
To examine the effects of combining non-invasive brain stimulation (NIBS) with neuromuscular electrical stimulation (NMES) on upper limb function in patients with a stroke.
Recent Findings
Of 580 articles, five studies met the eligibility criteria, involving 226 patients (mean age = 58.73 years), 45% of whom were female. Three studies considered “good” quality, one exhibited “excellent” quality, and one considered “poor” quality on the PEDro scale. There is heterogeneous evidence regarding the effects of the combined NIBS and NMES intervention on upper limb function in patients with a stroke.
Summary
The combination of NIBS with NMES might be a safe and well-tolerated intervention for patients with a stroke. However, the initial findings indicate that evidence regarding the effects of the combined NIBS and NMES intervention on upper limb function poststroke was limited. Further studies are strongly needed to understand the effects of the combined NIBS and NMES on upper limb outcomes and to identify the optimal treatment parameter.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- Sacco RL, Kasner SE, Broderick JP, et al. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44(7):2064–89. https://doi.org/10.1161/STR.0b013e318296aeca.Article PubMed Google Scholar
- Simpson LA, Hayward KS, McPeake M, Field TS, Eng JJ. Challenges of Estimating Accurate Prevalence of Arm Weakness Early After Stroke. Neurorehabil Neural Repair. 2021;35(10):871–9. https://doi.org/10.1177/15459683211028240.Article PubMed PubMed Central Google Scholar
- Grefkes C, Fink GR. Recovery from stroke: current concepts and future perspectives. Neurol Res Pract. 2020;2:17. https://doi.org/10.1186/s42466-020-00060-6.Article PubMed PubMed Central Google Scholar
- Hatem SM, Saussez G, Della Faille M, et al. Rehabilitation of Motor Function after Stroke: A Multiple Systematic Review Focused on Techniques to Stimulate Upper Extremity Recovery. Front Hum Neurosci. 2016;10:442. Published 2016 Sep 13. https://doi.org/10.3389/fnhum.2016.00442
- Nudo RJ, Plautz EJ, Frost SB. Role of adaptive plasticity in recovery of function after damage to motor cortex. Muscle Nerve. 2001;24(8):1000–19. https://doi.org/10.1002/mus.1104.Article CAS PubMed Google Scholar
- Simkins M, Kim H, Abrams G, Byl N, Rosen J. Robotic unilateral and bilateral upper-limb movement training for stroke survivors afflicted by chronic hemiparesis. IEEE Int Conf Rehabil Robot. 2013;2013:6650506. https://doi.org/10.1109/icorr.2013.6650506
- Krause T, Asseyer S, Taskin B, et al. The Cortical Signature of Central Poststroke Pain: Gray Matter Decreases in Somatosensory, Insular, and Prefrontal Cortices. Cereb Cortex. 2016;26(1):80–8. https://doi.org/10.1093/cercor/bhu177.Article CAS PubMed Google Scholar
- Chen G, Huang C, Liu Y, et al. Efficacy and safety of grain moxibustion in hemiplegia: A systematic review and meta-analysis protocol. Medicine (Baltimore). 2019;98(17):e15215. https://doi.org/10.1097/MD.0000000000015215.Article PubMed Google Scholar
- Hummel F, Cohen LG. Improvement of motor function with noninvasive cortical stimulation in a patient with chronic stroke. Neurorehabil Neural Repair. 2005;19(1):14–9. https://doi.org/10.1177/1545968304272698.Article PubMed Google Scholar
- Chipchase LS, Schabrun SM, Hodges PW. Peripheral electrical stimulation to induce cortical plasticity: a systematic review of stimulus parameters. Clin Neurophysiol. 2011;122(3):456–63. https://doi.org/10.1016/j.clinph.2010.07.025.Article CAS PubMed Google Scholar
- de Kroon JR, Ijzerman MJ, Lankhorst GJ, Zilvold G. Electrical stimulation of the upper limb in stroke: stimulation of the extensors of the hand vs. alternate stimulation of flexors and extensors. Am J Phys Med Rehabil. 2004;83(8):592–600. https://doi.org/10.1097/01.phm.0000133435.61610.55.Article PubMed Google Scholar
- Kristensen MGH, Busk H, Wienecke T. Neuromuscular electrical stimulation improves activities of daily living post stroke: a systematic review and meta-analysis. Arch Rehabil Res Clin Transl. 2021;4(1):100167. https://doi.org/10.1016/j.arrct.2021.100167
- Ward NS. Mechanisms underlying recovery of motor function after stroke. Postgrad Med J. 2005;81(958):510–4. https://doi.org/10.1136/pgmj.2004.030809.Article CAS PubMed PubMed Central Google Scholar
- Simonetti D, Zollo L, Milighetti S, et al. Literature review on the effects of tDCS Coupled with robotic therapy in post stroke upper limb rehabilitation. Front Hum Neurosci. 2017;11:268. https://doi.org/10.3389/fnhum.2017.00268.
- •• Alashram AR, Padua E, Romagnoli C, Raju M, Annino G. Effects of Repetitive transcranial magnetic stimulation on upper extremity spasticity Post-Stroke: A Systematic review. Physikalische Medizin Rehabilitationsmedizin Kurortmedizin. 2021. https://doi.org/10.1055/a-1691-9641. This study reported that combining rTMS with other rehabilitation interventions may show a superior effect in reducing the upper extremity spasticity compared with rTMS intervention alone.Article Google Scholar
- Alashram AR, Padua E, Aburub A, Raju M, Annino G. Transcranial direct current stimulation for upper extremity spasticity rehabilitation in stroke survivors: A systematic review of randomized controlled trials. PM R. 2023;15(2):222–34. https://doi.org/10.1002/pmrj.12804.Article PubMed Google Scholar
- Tedla JS, Sangadala DR, Reddy RS, Gular K, Kakaraparthi VN, Asiri F. Transcranial direct current stimulation (tDCS) effects on upper limb motor function in stroke: an overview review of the systematic reviews. Brain Inj. 2023;37(2):122–33. https://doi.org/10.1080/02699052.2022.2163289.Article PubMed Google Scholar
- Chen G, Lin T, Wu M, et al. Effects of repetitive transcranial magnetic stimulation on upper-limb and finger function in stroke patients: A systematic review and meta-analysis of randomized controlled trials. Front Neurol. 2022;13:940467. https://doi.org/10.3389/fneur.2022.940467.Article PubMed PubMed Central Google Scholar
- Lee JH, Jeun YJ, Park HY, Jung YJ. Effect of transcranial direct current stimulation combined with rehabilitation on arm and hand function in stroke patients: a systematic review and meta-analysis. Healthcare (Basel). 2021;9(12):1705. https://doi.org/10.3390/healthcare9121705
- Figlewski K, Blicher JU, Mortensen J, Severinsen KE, Nielsen JF, Andersen H. Transcranial Direct Current Stimulation Potentiates Improvements in Functional Ability in Patients With Chronic Stroke Receiving Constraint-Induced Movement Therapy. Stroke. 2017;48(1):229–32. https://doi.org/10.1161/STROKEAHA.116.014988.Article PubMed Google Scholar
- Dehem S, Gilliaux M, Lejeune T, et al. Effectiveness of a single session of dual-transcranial direct current stimulation in combination with upper limb robotic-assisted rehabilitation in chronic stroke patients: a randomized, double-blind, cross-over study. Int J Rehabil Res. 2018;41(2):138–45. https://doi.org/10.1097/MRR.0000000000000274.Article PubMed Google Scholar
- Gersner R, Kravetz E, Feil J, Pell G, Zangen A. Long-term effects of repetitive transcranial magnetic stimulation on markers for neuroplasticity: differential outcomes in anesthetized and awake animals. J Neurosci. 2011;31(20):7521–6. https://doi.org/10.1523/JNEUROSCI.6751-10.2011.Article CAS PubMed PubMed Central Google Scholar
- • Del Felice A, Daloli V, Masiero S, Manganotti P. Cathodal versus dual transcranial direct current stimulation for decreasing upper limb spasticity in chronic stroke individuals: a clinical and neurophysiological study [published correction appears in J Stroke Cerebrovasc Dis 2016;25(12):2932–41. https://doi.org/10.1016/j.jstrokecerebrovasdis.2016.08.008. This study demonstrated that cathodal tDCS is slightly more effective than dual tDCS in reducing distal upper limb spasticity in chronic poststroke subjects.
- • Hainaut K, Duchateau J. Neuromuscular electrical stimulation and voluntary exercise. Sports Med. 1992;14(2):100–13. https://doi.org/10.2165/00007256-199214020-00003(Thisstudyshowedthat. NMES effectively retards muscle wasting during denervation or immobilization and optimizes recovery of muscle strength during rehabilitation.Article CAS PubMed Google Scholar
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. https://doi.org/10.1136/bmj.n71
- Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100. https://doi.org/10.1371/journal.pmed.1000100.Article PubMed PubMed Central Google Scholar
- Macedo LG, Elkins MR, Maher CG, Moseley AM, Herbert RD, Sherrington C. There was evidence of convergent and construct validity of Physiotherapy Evidence Database quality scale for physiotherapy trials. J Clin Epidemiol. 2010;63(8):920–5. https://doi.org/10.1016/j.jclinepi.2009.10.005.Article PubMed Google Scholar
- Foley NC, Teasell RW, Bhogal SK, Speechley MR. Stroke Rehabilitation Evidence-Based Review: methodology. Top Stroke Rehabil. 2003;10(1):1–7.Article PubMed Google Scholar
- Du J, Wang S, Cheng Y, et al. Effects of neuromuscular electrical stimulation combined with repetitive transcranial magnetic stimulation on upper limb motor function rehabilitation in stroke patients with hemiplegia. Comput Math Methods Med. 2022;2022:9455428. https://doi.org/10.1155/2022/9455428
- Etoh S, Kawamura K, Tomonaga K, et al. Effects of concomitant neuromuscular electrical stimulation during repetitive transcranial magnetic stimulation before repetitive facilitation exercise on the hemiparetic hand. NeuroRehabilitation. 2019;45(3):323–9. https://doi.org/10.3233/NRE-192800.Article PubMed Google Scholar
- Wei YY, Koh CL, Hsu MJ, Lo SK, Chen CH, Lin JH. Effects of Transcranial Direct Current Stimulation Combined With Neuromuscular Electrical Stimulation on Upper Extremity Motor Function in Patients With Stroke. Am J Phys Med Rehabil. 2022;101(2):145–51. https://doi.org/10.1097/PHM.0000000000001759.Article PubMed Google Scholar
- Tosun A, Türe S, Askin A, et al. Effects of low-frequency repetitive transcranial magnetic stimulation and neuromuscular electrical stimulation on upper extremity motor recovery in the early period after stroke: a preliminary study. Top Stroke Rehabil. 2017;24(5):361–7. https://doi.org/10.1080/10749357.2017.1305644.Article PubMed Google Scholar
- Koyama S, Tanabe S, Warashina H, et al. NMES with rTMS for moderate to severe dysfunction after stroke. NeuroRehabilitation. 2014;35(3):363–8. https://doi.org/10.3233/NRE-141127.Article PubMed Google Scholar
- Di Pino G, Pellegrino G, Assenza G, et al. Modulation of brain plasticity in stroke: a novel model for neurorehabilitation. Nat Rev Neurol. 2014;10(10):597–608. https://doi.org/10.1038/nrneurol.2014.162.Article PubMed Google Scholar
- Boros K, Poreisz C, Münchau A, Paulus W, Nitsche MA. Premotor transcranial direct current stimulation (tDCS) affects primary motor excitability in humans. Eur J Neurosci. 2008;27(5):1292–300. https://doi.org/10.1111/j.1460-9568.2008.06090.x.Article PubMed Google Scholar
- Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol. 2000;527(Pt 3):633–9. https://doi.org/10.1111/j.1469-7793.2000.t01-1-00633.x.Article CAS PubMed PubMed Central Google Scholar
- Gartside IB. Mechanisms of sustained increases of firing rate of neurons in the rat cerebral cortex after polarization: reverberating circuits or modification of synaptic conductance? Nature. 1968;220(5165):382–3. https://doi.org/10.1038/220382a0.Article CAS PubMed Google Scholar
- Rossi S, Hallett M, Rossini PM, Pascual-Leone A. Safety of TMS Consensus Group. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol. 2009;120(12):2008–39. https://doi.org/10.1016/j.clinph.2009.08.016.Article PubMed PubMed Central Google Scholar
- Sanchis-Gomar F, Lopez-Lopez S, Romero-Morales C, Maffulli N, Lippi G, Pareja-Galeano H. Neuromuscular electrical stimulation: a new therapeutic option for chronic diseases based on contraction-induced myokine secretion. Front Physiol. 2019;10:1463. https://doi.org/10.3389/fphys.2019.01463
- Knutson JS, Fu MJ, Sheffler LR, Chae J. Neuromuscular Electrical Stimulation for Motor Restoration in Hemiplegia. Phys Med Rehabil Clin N Am. 2015;26(4):729–45. https://doi.org/10.1016/j.pmr.2015.06.002.Article PubMed PubMed Central Google Scholar
- Harris JE, Eng JJ. Strength training improves upper-limb function in individuals with stroke: a meta-analysis. Stroke. 2010;41(1):136–40. https://doi.org/10.1161/STROKEAHA.109.567438.Article PubMed Google Scholar
- Chae J. Neuromuscular electrical stimulation for motor relearning in hemiparesis. Phys Med Rehabil Clin N Am. 2003;14(1 Suppl):S93–109. https://doi.org/10.1016/s1047-9651(02)00051-7.Article PubMed Google Scholar
- Greve KR, Joseph CF, Berry BE, Schadl K, Rose J. Neuromuscular electrical stimulation to augment lower limb exercise and mobility in individuals with spastic cerebral palsy: a scoping review. Front Physiol. 2022;13:951899. https://doi.org/10.3389/fphys.2022.951899
- Guo Y, Phillips EB, Atherton PJ, Piasecki M. Molecular and neural adaptations to neuromuscular electrical stimulation; Implications for ageing muscle. Mech Ageing Dev. 2021;193:111402. https://doi.org/10.1016/j.mad.2020.111402.Article CAS PubMed PubMed Central Google Scholar
- Koseki T, Kudo D, Yoshida K, et al. Combined neuromuscular electrical stimulation and transcutaneous spinal direct current stimulation increases motor cortical plasticity in healthy humans. Front Neurosci. 2023;16:1034451. https://doi.org/10.3389/fnins.2022.1034451.Article PubMed PubMed Central Google Scholar
- Mollayeva T, Mollayeva S, Colantonio A. Traumatic brain injury: sex, gender and intersecting vulnerabilities. Nat Rev Neurol. 2018;14(12):711–22. https://doi.org/10.1038/s41582-018-0091-y.Article PubMed Google Scholar
- Ng YS, Tan KH, Chen C, Senolos GC, Koh GC. How Do Recurrent and First-Ever Strokes Differ in Rehabilitation Outcomes? Am J Phys Med Rehabil. 2016;95(10):709–17. https://doi.org/10.1097/PHM.0000000000000502.Article PubMed Google Scholar
- Perna R, Temple J. Rehabilitation Outcomes: Ischemic versus Hemorrhagic Strokes. Behav Neurol. 2015;2015:891651. https://doi.org/10.1155/2015/891651.Article PubMed PubMed Central Google Scholar
- Oosterveer DM, Wermer MJH, Volker G, Vlieland TPMV. Are there differences in long-term functioning and recovery between hemorrhagic and ischemic stroke patients receiving rehabilitation? J Stroke Cerebrovasc Dis. 2022;31(3):106294. https://doi.org/10.1016/j.jstrokecerebrovasdis.2021.106294.Article PubMed Google Scholar
- Salvadori E, Papi G, Insalata G, et al. Comparison between ischemic and hemorrhagic strokes in functional outcome at discharge from an intensive rehabilitation hospital. Diagnostics (Basel). 2020;11(1):38. https://doi.org/10.3390/diagnostics11010038
- Naghdi S, Ansari NN, Mansouri K, Hasson S. A neurophysiological and clinical study of Brunnstrom recovery stages in the upper limb following stroke. Brain Inj. 2010;24(11):1372–8. https://doi.org/10.3109/02699052.2010.506860.Article PubMed Google Scholar
- Longley V, Peters S, Swarbrick C, Bowen A. What factors affect clinical decision-making about access to stroke rehabilitation? A systematic review Clin Rehabil. 2019;33(2):304–16. https://doi.org/10.1177/0269215518808000.Article PubMed Google Scholar
- Hatem SM, Saussez G, Della Faille M, et al. Rehabilitation of Motor Function after Stroke: A Multiple Systematic Review Focused on Techniques to Stimulate Upper Extremity Recovery. Front Hum Neurosci. 2016;10:442. https://doi.org/10.3389/fnhum.2016.00442.Article CAS PubMed PubMed Central Google Scholar
- Cifu DX, Stewart DG. Factors affecting functional outcome after stroke: a critical review of rehabilitation interventions. Arch Phys Med Rehabil. 1999;80(5 Suppl 1):S35–9. https://doi.org/10.1016/s0003-9993(99)90101-6.Article CAS PubMed Google Scholar
- Simić-Panić D, Bošković K, Milićević M, et al. The Impact of Comorbidity on Rehabilitation Outcome after Ischemic Stroke. Acta Clin Croat. 2018;57(1):5–15. https://doi.org/10.20471/acc.2018.57.01.01.Article PubMed PubMed Central Google Scholar
- Tan M, Li H, Wang X. Analysis of the current status of rehabilitation motivation and its influencing factors in older adults with stroke: a cross-sectional study. Front Aging Neurosci. 2023;15:1186681. https://doi.org/10.3389/fnagi.2023.1186681
- Jung HY. Rehabilitation in subacute and chronic stage after stroke. In: Springer EBooks; 2017:351–360. https://doi.org/10.1007/978-981-10-1424-6_33
- van Lieshout ECC, van der Worp HB, Visser-Meily JMA, Dijkhuizen RM. Timing of Repetitive Transcranial Magnetic Stimulation Onset for Upper Limb Function After Stroke: A Systematic Review and Meta-Analysis. Front Neurol. 2019;10:1269. https://doi.org/10.3389/fneur.2019.01269.Article PubMed PubMed Central Google Scholar
- Lee JH, Jeun YJ, Park HY, Jung YJ. Effect of Transcranial Direct Current Stimulation Combined with Rehabilitation on Arm and Hand Function in Stroke Patients: A Systematic Review and Meta-Analysis. Healthcare (Basel). 2021;9(12):1705. https://doi.org/10.3390/healthcare9121705.Article PubMed Google Scholar
- Obayashi S, Saito H. Neuromuscular Stimulation as an Intervention Tool for Recovery from Upper Limb Paresis after Stroke and the Neural Basis. Appl Sci. 2022;12(2):810. https://doi.org/10.3390/app12020810.Article CAS Google Scholar
- Raghavan P. Upper Limb Motor Impairment After Stroke. Phys Med Rehabil Clin N Am. 2015;26(4):599–610. https://doi.org/10.1016/j.pmr.2015.06.008.Article PubMed PubMed Central Google Scholar
- Wu L, Subramanian N, Abdulrahman MD, Liu C, Pawar KS. Short-term versus long-term benefits: Balanced sustainability framework and research propositions. Sustain Prod Consum. 2017;11:18–30. https://doi.org/10.1016/j.spc.2016.09.003.Article Google Scholar
- Hara Y. Brain plasticity and rehabilitation in stroke patients. J Nippon Med Sch. 2015;82(1):4–13. https://doi.org/10.1272/jnms.82.4.Article PubMed Google Scholar
- Lieshout ECCV, van de Port IG, Dijkhuizen RM, Visser-Meily JMA. Does upper limb strength play a prominent role in health-related quality of life in stroke patients discharged from inpatient rehabilitation? Top Stroke Rehabil. 2020;27(7):525–33. https://doi.org/10.1080/10749357.2020.1738662.Article PubMed Google Scholar
- Martin S, Cordeiro L, Richardson P, Davis S, Tartaglia N. The Association of Motor Skills and Adaptive Functioning in XXY/Klinefelter and XXYY Syndromes. Phys Occup Ther Pediatr. 2019;39(4):446–59. https://doi.org/10.1080/01942638.2018.1541040.Article PubMed Google Scholar
- Jenkinson C, Fitzpatrick R, Crocker H, Peters M. The Stroke Impact Scale: validation in a UK setting and development of a SIS short form and SIS index. Stroke. 2013;44(9):2532–5. https://doi.org/10.1161/STROKEAHA.113.001847.Article PubMed Google Scholar
- Sears ED, Chung KC. Validity and responsiveness of the Jebsen-Taylor Hand Function Test. J Hand Surg Am. 2010;35(1):30–7. https://doi.org/10.1016/j.jhsa.2009.09.008.Article PubMed Google Scholar
- Feys P, Lamers I, Francis G, et al. The Nine-Hole Peg Test as a manual dexterity performance measure for multiple sclerosis. Mult Scler. 2017;23(5):711–20. https://doi.org/10.1177/1352458517690824.Article PubMed PubMed Central Google Scholar
- Johnson D, Harris JE, Stratford P, Richardson J. Interrater Reliability of Three Versions of the Chedoke Arm and Hand Activity Inventory. Physiother Can. 2018;70(2):133–40. https://doi.org/10.3138/ptc.2016-70.Article PubMed PubMed Central Google Scholar
- Uswatte G, Taub E, Morris D, Vignolo M, McCulloch K. Reliability and validity of the upper-extremity Motor Activity Log-14 for measuring real-world arm use. Stroke. 2005;36(11):2493–6. https://doi.org/10.1161/01.STR.0000185928.90848.2e.Article PubMed Google Scholar
- Egger M, Smith GD. meta-analysis bias in location and selection of studies. BMJ. 1998;316(7124):61–6. https://doi.org/10.1136/bmj.316.7124.61.Article CAS PubMed PubMed Central Google Scholar
[WEB] NEW Royal College of Physicians Stroke Guidelines
Posted by Kostas Pantremenos in Functional Electrical Stimulation (FES), REHABILITATION on April 10, 2023
| The Royal College of Physicians have published their updated National Clinical Guidelines for Stroke for the UK and Ireland. This replaces the 2016 edition and are accredited by NICE. The new guidelines have improved recommendations for rehabilitation, including a recommended 3 hours of therapy a day within the recovery stage. They also state that people with stroke should be considered to have the potential to benefit from rehabilitation at any point after their stroke. A greater emphasis is put on the need for intensity and repetition to maximise the benefit from therapeutic interventions. In support of this, the role of FES / NMES is recognised and its use is recommended in several areas, including shoulder subluxation pain, muscle weakness, retraining of movement and supporting walking. The exception is the use of NMES for control of spasticity, although it is recommended in conjunction with Botox or where splinting is insufficient. Listed below are the relevant sections regarding FES / NMES. You can download the whole report for free at https://www.strokeguideline.org/ 4.17 Motor impairment People with stroke who are unable to exercise against gravity independently should be considered for adjuncts to exercise (such as neuromuscular or functional electrical stimulation), to support participation in exercise training. [2023] Electrical stimulation Electrical stimulation has been used as an adjunctive treatment for the upper limb for many years. The most common form is therapeutic or cyclical electrical stimulation (also known as neuromuscular electrical stimulation [NMES]) to the wrist and finger extensors, which stimulates the muscles to contract in order to improve weakness and reduce motor impairment. [2023] 4.18 Arm function People with wrist and finger weakness which limits function after stroke should be considered for functional electrical stimulation applied to the wrist and finger extensors, as an adjunct to conventional therapy. Stimulation protocols should be individualised to the person’s presentation and tolerance, and the person with stroke, their family/carers and clinicians in all settings should be trained in the safe application and use of electrical stimulation devices. [2023] 4.21 Falls and fear of falling People with stroke and limitations of dorsiflexion or ankle instability causing impaired balance and risk or fear of falling should be considered for referral to orthotics for an ankle-foot orthosis and/or functional electrical stimulation. The person with stroke, their family/carers and clinicians in all settings should be trained in the safe use and application of orthoses and electrical stimulation devices. [2023] 4.22 Walking People with stroke with limited ankle/foot stability or limited dorsiflexion (‘foot drop’) that impedes mobility or confidence should be offered an ankle-foot orthosis (using a lightweight, flexible orthosis in the first instance) or functional electrical stimulation to improve walking and balance, including referral to orthotics if required. – Any orthosis or electrical stimulation device should be evaluated and individually fitted before long-term use. – The person with stroke, their family/carers and clinicians in all settings should be trained in the safe application and use of orthoses and electrical stimulation devices. – People using an orthosis after stroke should be educated about the risk of pressure damage from their orthosis, especially if sensory loss is present in addition to weakness. Services should provide timely access for orthotic repairs and adaptations. [2023] Stroke services should have local protocols and agreements in place to ensure specialist assessment, evaluation and follow-up is available for long-term functional electrical stimulation use. [2023] 4.23.3 Shoulder subluxation and pain People with inferior shoulder subluxation within 6 months of hemiplegic stroke should be considered for neuromuscular electrical stimulation, unless contraindicated. The stimulation protocol should be individualised to the person’s presentation and tolerance. The person with stroke, their family/carers and clinicians in all settings should be trained in the safe application and use of electrical stimulation devices. [2023] 4.24 Spasticity and contractures People with spasticity in the upper or lower limbs after stroke should not be treated with electrical stimulation to reduce spasticity. [2023] People with spasticity in their wrist or fingers who have been treated with botulinum toxin may be considered for electrical stimulation (cyclical/neuromuscular electrical stimulation) after the injection to maintain range of movement and/or to provide regular stretching as an adjunct to splinting or when splinting is not tolerated. [2023] 4.26 Swallowing People with dysphagia after stroke may be considered for neuromuscular electrical stimulation as an adjunct to behavioural rehabilitation where the device is available and it can be delivered by a trained healthcare professional. [2023] Kind regards Paul Taylor Clinical Director |
[WEB] Motor Recovery of Upper Limbs After Stroke
Posted by Kostas Pantremenos in Paretic Hand on April 18, 2022
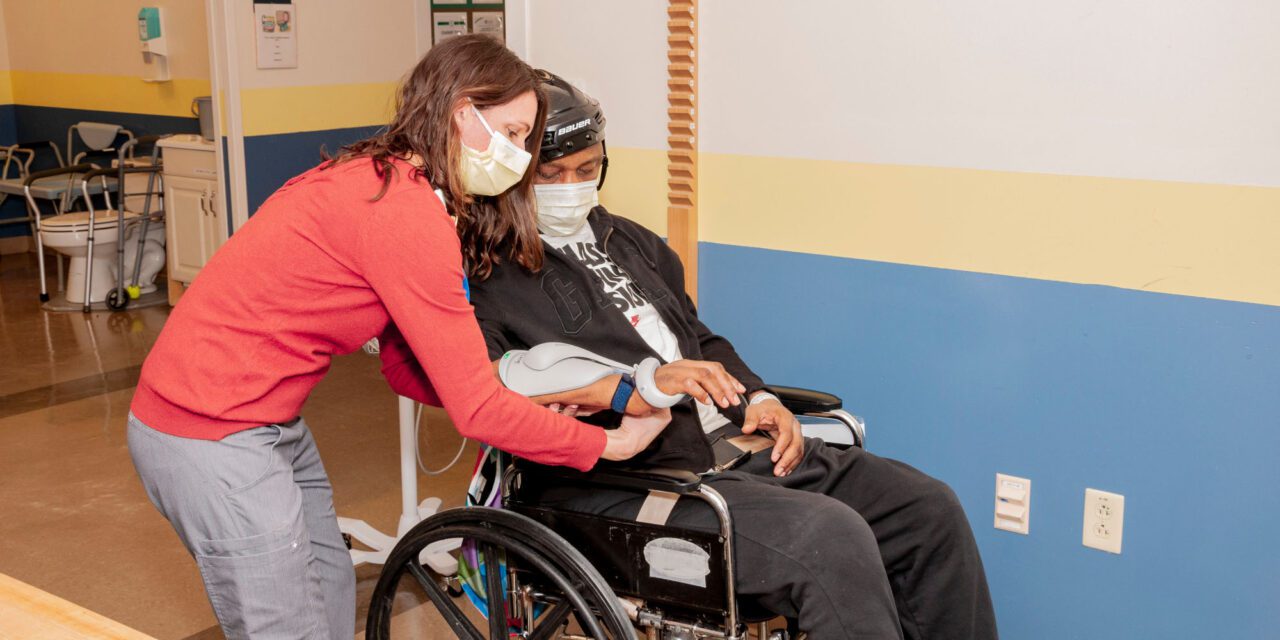
How newer technologies can help drive motor recovery of upper extremities in stroke patients.
By Megan Palmer, OTR/L
The magic of neuroplasticity following a stroke has long been a phenomenon of neurological recovery. Thousands of studies have been performed to understand specific treatment interventions that drive this neurological change. How is it that damaged or dead brain tissue following a stroke can be circumvented to allow for new nerve pathways that result in motor recovery?
For a considerable time, stroke was known as a focal disease at the area of infarct, but the neurological deficits that present themselves tend to have an impact on areas distal to the damaged brain tissue, if not to the entire brain. The localized stroke may result in deficits affecting all areas of function including motor, sensory, visual, cognitive, speech, and emotional.
A neuroimaging technique called diffusion weighted imaging (DWI) provides the ability to analyze in vivo white matter bundles and show how areas of the brain are interconnected. DWI can provide a map of routes for reorganization of neural signals/impulses that can be a highway for neuroplasticity.1
With the information that associated centers of the brain can be simultaneously affected by an infarct in a distal area, neuroscientists strive to identify how treatment interventions related to stroke can be utilized to drive neurological change. The use of Functional Magnetic Resonance Imaging (fMRI), in addition to other neuroimaging technologies, can provide distinctive information on the networking of the human brain and what areas are “lighting up” when certain motor activities are performed. Essentially, fMRI is able to identify areas of increased blood flow associated with active neurons requiring an increase in oxygen supply. This connection can be interpreted as the brain adapting to discover new ways to recover normal motor function.2

Guiding Principles in Motor Recovery
The most common deficit after stroke is hemiparesis of the upper extremity (UE), contralateral to the site of the brain infarct. Eighty-five percent of stroke survivors experience hemiparesis and 65% result in functional limitations. These are striking numbers that directly impact a patient’s quality of life and determine his or her level of dependence on others.3
Utilizing the methodology that neural recovery is possible via fMRI data can guide therapeutic interventions ensuring high-quality, high dosage, high repetitions of normal movement patterns to aid in the motor recovery of the hemiplegic UE.Research has shown there is no specific prescription or protocol for rehabilitation after stroke, but there are principles to guide current practice:
• Specificity—Need to be direct with treatment intervention to enhance neuroplasticity; task-specific training.
• Repetition—Repeat movement patterns; practice in the way that is going to be effective for the rest of their lives (do not teach compensatory movements with the affected UE).
• Intensity—It matters; work until failure; take to the point that they cannot perform the activity anymore; push them, challenge them; the demand is what is going to drive change in the brain.
• Salience—Has to mean something to the patient, they have to buy-in, physiological changes in the brain are to a greater degree when activity is purposeful.
• Allow for patient error—Neuroplastic changes occur in the midst of errors, error-correction, failure, near-misses, and exploration.
• Utilize an enriched environment—Integrate sensory and intellectual stimulation.
Neurological change occurs when you practice the next level, add variables, and provide the just-right challenge for the patient. This rewiring of the brain does not occur in stagnant environments, with the practice of already mastered skills or with familiar tasks. Treatment interventions need to be tailored to the patient’s goals and relate to movements that carry over to functional tasks.4
Due to the variability of stroke paralysis that occurs in each patient and the limiting factors including spasticity and tone, it is stated that a variety of conventional methods and technologies should be used to treat hemiplegia post-stroke. Additional technologies have proven to be an effective adjunct to conventional stroke rehabilitation interventions. Electrical stimulation, functional rehabilitation systems, myoelectric devices, and virtual reality are all examples of technologies that aid in stroke rehabilitation of the upper extremity.5
Neuromuscular Electrical Stimulation (NMES) in Motor Recovery
NMES is used to target specific muscle groups for patients with hemiplegia following stroke and promote motor facilitation. It provides an alternate route to gain maximum repetitions of an otherwise paralyzed muscle.
NMES utilizes an electric current that produces a contraction of a muscle with intact lower motor neuron connections. The NMES device allows for adjustments to be made with frequency, pulse width and amplitude that produce a waveform resulting in a visible muscle contraction. The variability of electrode placement and multiple leads allow for the ability to stimulate more than one muscle group at a time. This can assist with providing adequate contractions in the shoulder muscles to decrease subluxation and distal muscles of the upper extremity to produce repetitive stimulation.
Research is inconclusive on exactly how effective electrical stimulation is to recovery of a hemiparetic upper extremity, but it is one of the most common and favorably mentioned in stroke rehabilitation practices.6
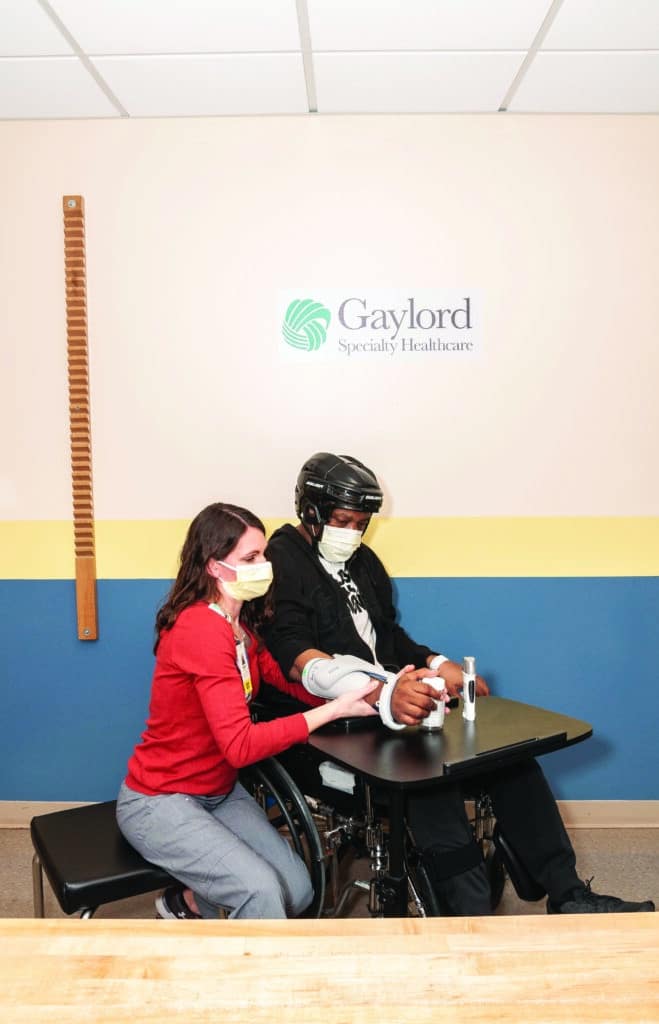
Functional Electrical Stimulation Unit for the Hand
A wireless hand rehabilitation system that provides electrical stimulation to the wrist and digit flexors and extensors is a device that is often used by inpatient and outpatient therapists at Gaylord Specialty Healthcare, where I work.
The device is applied to the forearm and has five electrodes that stimulate the extensor digitorum, extensor pollicis brevis, flexor digitorum superficialis, flexor pollicis longus, and thenar muscles to promote gross grasp and release.7 The unit allows the UE to be incorporated into a functional grasp/release task such as holding a cup and bringing it to the mouth or holding a jar while the unaffected UE opens it. These functions can open a world of opportunity for a patient with hemiparesis if used in their daily life. Due to the repetitive nature and neuromuscular stimulation, this technology also has been favored to aid in neurological recovery.
Myoelectric Upper Limb Orthoses for Stroke Rehab
A neuro-robotic arm brace was designed to assist in motor recovery of the upper extremity following a stroke. Devices like this fit over the elbow and secure on the proximal humerus and distal forearm. Sensors are built into the cuffs that align over the biceps and triceps to detect electromyographic (EMG) signals when the patient initiates movement into elbow flexion or extension. This triggers the device to assist in completing the full range of motion in that particular plane. This allows repetitive biceps/triceps movement related to function that assists in rebuilding the neural pathways to regain control of the affected arm.8
There are also devices adapted from this model with two additional sensors on the forearm to sense an initiation of wrist/hand flexors and extensors to promote multi-joint functional movements. These newer devices were designed for daily use and are lightweight for patients’ comfort while performing daily activities or job tasks.9 Studies have shown that the use of portable, myoelectric elbow-wrist-hand orthoses have promising effects on improved motor recovery of the upper extremity, but are vague on reporting the long-term lasting effects of motor return when the device is taken off.10
Arm and Hand Rehabilitation Systems
Functional rehabilitation systems for the upper extremities utilize movement biofeedback and the patient’s current range of motion and strength to enhance and drive neurological change.
Gaylord recently invested in a device of this nature combined with virtual gaming to promote neurological return of the upper extremity. In the initial trial period, patients have shown positive engagement in the gaming device when otherwise their participation was minimal. Their attention is focused on the game instead of on the actual number of repetitions they are performing.
The device is placed on the affected UE with the hinge lock at the lateral elbow. The software assesses patients’ active range of motion into elbow flexion and extension and utilizes those movements to promote repetitive exercise in a fun way.
A hand rehabilitation system is the same concept as the arm device, but instead an ergonomic wearable glove is applied to the patient’s affected hand to utilize their current active hand and wrist motion and capitalize on it.11
Virtual Reality
While research is limited on the effects of virtual reality technology improving hemiplegia in stroke patients, we know that it provides an incentive to perform a repetitive exercise when utilizing a gaming system. In the Sevgi, et al12 study and the Kim13 study that examine stroke patients in a control group receiving conventional therapy and an experimental group receiving conventional and use of video games, improvements of UE function were significantly higher in a few of the UE assessments. In fact, the Kim study abstract states that “Stroke patients who completed the additional training using virtual reality games showed significantly greater improvement in their daily living activities than those who only received traditional rehabilitation therapy.”13 According to a study by Kiper et al,14 reinforced feedback in virtual environment (RFVE) therapy combined with conventional rehabilitation treatment promotes better outcomes for upper extremities than the same amount of conventional rehabilitation, for all types of stroke.
Conclusion
In summary, the majority of studies involving stroke rehabilitation technologies state that they may have positive effects on UE function in stroke patients, but additional studies are needed with greater sample sizes and more precise treatment prescriptions. Specifics are needed to identify how much and how long to utilize the technologies to make the greatest significant differences in UE recovery. In addition, the majority of studies do not utilize patients in their first six months post-stroke. Typically, the greatest neurological recovery is seen in the first three-to-six months following a stroke, which would be the ideal time to investigate the effects of conventional versus technological interventions.
In conclusion, review of the studies mentioned with the use of these technologies, including virtual reality, do not show any negative effects; they only contribute to the patient’s motor recovery and function of UE hemiparesis and would overall complement stroke rehabilitation. RM
Megan Palmer, OTR/L, is a Level III inpatient occupational therapist for Gaylord Specialty Healthcare in Wallingford, Conn. Palmer received her Bachelor of Rehabilitation and Disabilities Studies with a minor in Psychology and her Master’s Degree in Occupational Therapy from Springfield College and has been working with patients with traumatic brain injury and stroke for 15 years.
References
1 Guggisberg AG, Koch PJ, Hummel FC, Buetefisch CM. Brain networks and their relevance for stroke rehabilitation. Clin Neurophysiol. 2019;130(7):1098-1124. doi: 10.1016/j.clinph.2019.04.004
2 Crofts A, Kelly ME, Gibson CL. Imaging Functional Recovery Following Ischemic Stroke: Clinical and Preclinical fMRI Studies. J Neuroimaging. 2020;30(1):5-14. doi: 10.1111/jon.12668
3 Gurbuz N, Afsar SI, Ayaş S, Cosar SN. Effect of mirror therapy on upper extremity motor function in stroke patients: a randomized controlled trial. J Phys Ther Sci. 2016;28(9):2501-2506. doi: 10.1589/jpts.28.2501
4 Green, M. (Aug 2021). PESI, Inc. Stroke Rehab Master Class – Best Practices for Improved Outcomes. Igniting Neuroplasticity after Stroke: Breakthroughs for Improving Motor Recovery.
5 Ward NS, Brander F, Kelly K. Intensive upper limb neurorehabilitation in chronic stroke: outcomes from the Queen Square programme. Journal of Neurology, Neurosurgery & Psychiatry. 2019;90(5):498-506.
6 Knutson JS, Fu MJ, Sheffler LR, Chae J. Neuromuscular Electrical Stimulation for Motor Restoration in Hemiplegia. Phys Med Rehabil Clin N Am. 2015;26(4):729-745. doi: 10.1016/j.pmr.2015.06.002
7 Takeda K, Tanino G, Miyasaka H. Review of devices used in neuromuscular electrical stimulation for stroke rehabilitation. Med Devices (Auckl). 2017;10:207-213. Published 2017 Aug 24. doi: 10.2147/MDER.S123464
8 Wasielewski D. 2010, April. Myomo Robotic Therapy Device. StrokeNetwork Newsletter. Retrieved from Stroke Network: http://strokenetwork.org/newsletter/therapies/myomo.htm
9 Demaitre E. (2019, June 7). Myomo scales up production, training for MyoPro device for upper-body mobility.
10 Peters HT, Page SJ, Persch A. Giving Them a Hand: Wearing a Myoelectric Elbow-Wrist-Hand Orthosis Reduces Upper Extremity Impairment in Chronic Stroke. Arch Phys Med Rehabil. 2017;98(9):1821-1827. doi: 10.1016/j.apmr.2016.12.016
11 MediTouch. (2018). MediTouch HandTutor. Retrieved from MediTouch Homepage: https://meditouch.co.il/products/handtutor/
12 Ikbali Afsar S, Mirzayev I, Umit Yemisci O, Cosar Saracgil SN. Virtual Reality in Upper Extremity Rehabilitation of Stroke Patients: A Randomized Controlled Trial. J Stroke Cerebrovasc Dis. 2018;27(12):3473-3478. doi: 10.1016/j.jstrokecerebrovasdis.2018.08.007
13 Kim JH. Effects of a virtual reality video game exercise program on upper extremity function and daily living activities in stroke patients. J Phys Ther Sci. 2018;30(12):1408-1411. doi: 10.1589/jpts.30.1408
14 Kiper P, Szczudlik A, Agostini M, et al. Virtual Reality for Upper Limb Rehabilitation in Subacute and Chronic Stroke: A Randomized Controlled Trial. Arch Phys Med Rehabil. 2018;99(5):834-842.e4. doi: 10.1016/j.apmr.2018.01.023
[ARTICLE] Neuromuscular Electrical Stimulation Improves Activities of Daily Living Post Stroke: A Systematic Review and Meta-analysis – Full Text
Posted by Kostas Pantremenos in REHABILITATION on April 10, 2022
Abstract
Objectives
(1) To elucidate the effectiveness of neuromuscular electrical stimulation (NMES) toward improving activities of daily living (ADL) and functional motor ability post stroke and (2) to investigate the influence of paresis severity and the timing of treatment initiation for the effectiveness of NMES.
Data Sources
PubMed, MEDLINE, Embase, Physiotherapy Evidence Database (PEDro) and Cochrane Library searched for relevant articles from database inception to May 2020.
Study Selection
The inclusion criteria were randomized controlled trials exploring the effect of NMES toward improving ADL or functional motor ability in survivors of stroke. The search identified 6064 potential articles with 20 being included.
Data Extraction
Two independent reviewers conducted the data extraction. Methodological quality was assessed using the PEDro scale and the Cochrane Risk of Bias Tool.
Data Synthesis
Data from 428 and 659 participants (mean age, 62.4 years; 54% male) for outcomes of ADL and functional motor ability, respectively, were pooled in a random-effect meta-analysis. The analysis revealed a significant positive effect of NMES toward ADL (standardized mean difference [SMD], 0.41; 95% CI, 0.14-0.67; P=.003), whereas no effect on functional motor ability was evident. Subgroup analyses showed that application of NMES in the subacute stage (SMD, 0.44; 95% CI, 0.09-0.78; P=.01) and in the upper extremity (SMD, 0.34; 95% CI, 0.04-0.64; P=.02) improved ADL, whereas a beneficial effect was observed for functional motor abilities in patients with severe paresis (SMD, 0.41; 95% CI, 0.12-0.70; P=.005).
Conclusions
The results of the present meta-analysis are indicative of potential beneficial effects of NMES toward improving ADL post stroke, whereas the potential for improving functional motor ability appears less clear. Furthermore, subgroup analyses indicated that NMES application in the subacute stage and targeted at the upper extremity is efficacious for ADL rehabilitation and that functional motor abilities can be positively affected in patients with severe paresis.
List of abbreviations: ADL, activities of daily living; BRS, Brunnstrom recovery stages; CROB, Cochrane risk of bias; EMG, electromyogram; ES, electrical stimulation; FES, functional electrical stimulation; MMT, manual muscle test; NIHSS, National Institutes of Health Stroke Scale; NMES, neuromuscular electrical stimulation; PEDro, Physiotherapy Evidence Database; SMD, standardized mean difference; TES, therapeutic electrical stimulation
——————————–
The global incidence of stroke is in the order of 13.7 million annually1 and is a clinical condition typically associated with limb paresis2 secondary to compromised function of upper motor neurons and associated neural pathways, with loss of locomotor function and the ability to perform activities of daily living (ADL) being functional manifestations hereof.3, 4, 5 Although recent medico-scientific advances within the fields of thrombolysis6,7 and thrombectomy8 have spurred major changes to the treatment of acute ischemic stroke, stroke remains a leading cause of disability,1 and effective rehabilitation modalities are thus of utmost importance.
In the newest Clinical Guidelines for Stroke Management9 and Guidelines for Adult Stroke Rehabilitation and Recovery,10 the rehabilitation modality of electrical stimulation (ES) is recommended as a supplementary therapy alongside the standard care modalities. ES can be broadly categorized into functional electrical stimulation (FES) and therapeutic electrical stimulation (TES). The primary difference between these 2 ES modalities is the degree of patient involvement; TES is administered with the patient completely passive or performing isolated muscle contractions, whereas FES is superimposed onto voluntary contractions while the patient is performing functional tasks such as walking, rising from a chair, or stair climbing.11,12 As alluded by Kroon et al,13 TES can be further subcategorized into neuromuscular electrical stimulation (NMES), electromyogram (EMG)-triggered ES, positional feedback stimulation training, and transcutaneous electrical nerve stimulation. In EMG-triggered and positional feedback stimulation, the electrical current is administered in response to the patient performing a minor contraction or movement, respectively, whereas NMES is administered according to a preprogrammed scheme and hence is received passively.11,14,15 Evidence suggests that NMES has the ability to strengthen muscles,16,17 reduce spasticity,10 increase excitability of corticospinal neural pathways,18 and augment neuroplasticity.19,20 Furthermore, when ES is administered prior to or after voluntary contractions (eg, NMES) in persons without stroke, it has been demonstrated to be more effective in developing functional motor abilities than both voluntary contractions performed simultaneously with stimulation and voluntary contractions performed in isolation.20,21 The apparent superiority could be governed by a cumulative effect of the 2 types of contractions and/or because of the unique motor drives associated with each type of contraction.21,22
According to the International Classification of Functioning, Disability, and Health, poststroke rehabilitation is a complex process that can be viewed in the context of function, activity, and participation domains.23 The activity domain encompasses the full range of life areas from a performance and capacity point of view, the performance level describes an individual’s abilities in the actual context in which they live (ADL), and the capacity level entails the ability to execute a specific task or action in a standard environment (functional motor ability).23 ADL reflect the level of disability in daily life and are therefore thought of as the most clinically relevant outcomes in assessing poststroke recovery,24 whereas functional motor abilities are viewed as good surrogate outcomes.
A number of systematic reviews concerning the effectiveness of ES toward regaining overall activity performance post stroke have been published, including 3 Cochrane reviews.17,25,26 However, the majority of said reviews have pooled studies with a variety of ES methods16,25,27, 28, 29, 30, 31 or investigated other specific aspects of ES, typically FES.24,32, 33, 34 In contrast to previous systematic reviews, the aim of the present systematic review and meta-analysis was to elucidate the effectiveness of NMES in improving ADL and functional motor ability post stroke and additionally analyze data according to onset of NMES administration post stroke and paresis severity, which in our opinion are important additions to the stroke rehabilitation literature. […]
[WEB] Motor Recovery of Upper Limbs After Stroke
Posted by Kostas Pantremenos in Functional Electrical Stimulation (FES), Neuroplasticity, Paretic Hand, REHABILITATION, Virtual reality rehabilitation on April 4, 2022
Posted by Debbie Overman

How newer technologies can help drive motor recovery of upper extremities in stroke patients.
By Megan Palmer, OTR/L
The magic of neuroplasticity following a stroke has long been a phenomenon of neurological recovery. Thousands of studies have been performed to understand specific treatment interventions that drive this neurological change. How is it that damaged or dead brain tissue following a stroke can be circumvented to allow for new nerve pathways that result in motor recovery?
For a considerable time, stroke was known as a focal disease at the area of infarct, but the neurological deficits that present themselves tend to have an impact on areas distal to the damaged brain tissue, if not to the entire brain. The localized stroke may result in deficits affecting all areas of function including motor, sensory, visual, cognitive, speech, and emotional.
A neuroimaging technique called diffusion weighted imaging (DWI) provides the ability to analyze in vivo white matter bundles and show how areas of the brain are interconnected. DWI can provide a map of routes for reorganization of neural signals/impulses that can be a highway for neuroplasticity.1
With the information that associated centers of the brain can be simultaneously affected by an infarct in a distal area, neuroscientists strive to identify how treatment interventions related to stroke can be utilized to drive neurological change. The use of Functional Magnetic Resonance Imaging (fMRI), in addition to other neuroimaging technologies, can provide distinctive information on the networking of the human brain and what areas are “lighting up” when certain motor activities are performed. Essentially, fMRI is able to identify areas of increased blood flow associated with active neurons requiring an increase in oxygen supply. This connection can be interpreted as the brain adapting to discover new ways to recover normal motor function.2

Guiding Principles in Motor Recovery
The most common deficit after stroke is hemiparesis of the upper extremity (UE), contralateral to the site of the brain infarct. Eighty-five percent of stroke survivors experience hemiparesis and 65% result in functional limitations. These are striking numbers that directly impact a patient’s quality of life and determine his or her level of dependence on others.3
Utilizing the methodology that neural recovery is possible via fMRI data can guide therapeutic interventions ensuring high-quality, high dosage, high repetitions of normal movement patterns to aid in the motor recovery of the hemiplegic UE.Research has shown there is no specific prescription or protocol for rehabilitation after stroke, but there are principles to guide current practice:
• Specificity—Need to be direct with treatment intervention to enhance neuroplasticity; task-specific training.
• Repetition—Repeat movement patterns; practice in the way that is going to be effective for the rest of their lives (do not teach compensatory movements with the affected UE).
• Intensity—It matters; work until failure; take to the point that they cannot perform the activity anymore; push them, challenge them; the demand is what is going to drive change in the brain.
• Salience—Has to mean something to the patient, they have to buy-in, physiological changes in the brain are to a greater degree when activity is purposeful.
• Allow for patient error—Neuroplastic changes occur in the midst of errors, error-correction, failure, near-misses, and exploration.
• Utilize an enriched environment—Integrate sensory and intellectual stimulation.
Neurological change occurs when you practice the next level, add variables, and provide the just-right challenge for the patient. This rewiring of the brain does not occur in stagnant environments, with the practice of already mastered skills or with familiar tasks. Treatment interventions need to be tailored to the patient’s goals and relate to movements that carry over to functional tasks.4
Due to the variability of stroke paralysis that occurs in each patient and the limiting factors including spasticity and tone, it is stated that a variety of conventional methods and technologies should be used to treat hemiplegia post-stroke. Additional technologies have proven to be an effective adjunct to conventional stroke rehabilitation interventions. Electrical stimulation, functional rehabilitation systems, myoelectric devices, and virtual reality are all examples of technologies that aid in stroke rehabilitation of the upper extremity.5
Neuromuscular Electrical Stimulation (NMES) in Motor Recovery
NMES is used to target specific muscle groups for patients with hemiplegia following stroke and promote motor facilitation. It provides an alternate route to gain maximum repetitions of an otherwise paralyzed muscle.
NMES utilizes an electric current that produces a contraction of a muscle with intact lower motor neuron connections. The NMES device allows for adjustments to be made with frequency, pulse width and amplitude that produce a waveform resulting in a visible muscle contraction. The variability of electrode placement and multiple leads allow for the ability to stimulate more than one muscle group at a time. This can assist with providing adequate contractions in the shoulder muscles to decrease subluxation and distal muscles of the upper extremity to produce repetitive stimulation.
Research is inconclusive on exactly how effective electrical stimulation is to recovery of a hemiparetic upper extremity, but it is one of the most common and favorably mentioned in stroke rehabilitation practices.6

Functional Electrical Stimulation Unit for the Hand
A wireless hand rehabilitation system that provides electrical stimulation to the wrist and digit flexors and extensors is a device that is often used by inpatient and outpatient therapists at Gaylord Specialty Healthcare, where I work.
The device is applied to the forearm and has five electrodes that stimulate the extensor digitorum, extensor pollicis brevis, flexor digitorum superficialis, flexor pollicis longus, and thenar muscles to promote gross grasp and release.7 The unit allows the UE to be incorporated into a functional grasp/release task such as holding a cup and bringing it to the mouth or holding a jar while the unaffected UE opens it. These functions can open a world of opportunity for a patient with hemiparesis if used in their daily life. Due to the repetitive nature and neuromuscular stimulation, this technology also has been favored to aid in neurological recovery.
Myoelectric Upper Limb Orthoses for Stroke Rehab
A neuro-robotic arm brace was designed to assist in motor recovery of the upper extremity following a stroke. Devices like this fit over the elbow and secure on the proximal humerus and distal forearm. Sensors are built into the cuffs that align over the biceps and triceps to detect electromyographic (EMG) signals when the patient initiates movement into elbow flexion or extension. This triggers the device to assist in completing the full range of motion in that particular plane. This allows repetitive biceps/triceps movement related to function that assists in rebuilding the neural pathways to regain control of the affected arm.8
There are also devices adapted from this model with two additional sensors on the forearm to sense an initiation of wrist/hand flexors and extensors to promote multi-joint functional movements. These newer devices were designed for daily use and are lightweight for patients’ comfort while performing daily activities or job tasks.9 Studies have shown that the use of portable, myoelectric elbow-wrist-hand orthoses have promising effects on improved motor recovery of the upper extremity, but are vague on reporting the long-term lasting effects of motor return when the device is taken off.10
Arm and Hand Rehabilitation Systems
Functional rehabilitation systems for the upper extremities utilize movement biofeedback and the patient’s current range of motion and strength to enhance and drive neurological change.
Gaylord recently invested in a device of this nature combined with virtual gaming to promote neurological return of the upper extremity. In the initial trial period, patients have shown positive engagement in the gaming device when otherwise their participation was minimal. Their attention is focused on the game instead of on the actual number of repetitions they are performing.
The device is placed on the affected UE with the hinge lock at the lateral elbow. The software assesses patients’ active range of motion into elbow flexion and extension and utilizes those movements to promote repetitive exercise in a fun way.
A hand rehabilitation system is the same concept as the arm device, but instead an ergonomic wearable glove is applied to the patient’s affected hand to utilize their current active hand and wrist motion and capitalize on it.11
Virtual Reality
While research is limited on the effects of virtual reality technology improving hemiplegia in stroke patients, we know that it provides an incentive to perform a repetitive exercise when utilizing a gaming system. In the Sevgi, et al12 study and the Kim13 study that examine stroke patients in a control group receiving conventional therapy and an experimental group receiving conventional and use of video games, improvements of UE function were significantly higher in a few of the UE assessments. In fact, the Kim study abstract states that “Stroke patients who completed the additional training using virtual reality games showed significantly greater improvement in their daily living activities than those who only received traditional rehabilitation therapy.”13 According to a study by Kiper et al,14 reinforced feedback in virtual environment (RFVE) therapy combined with conventional rehabilitation treatment promotes better outcomes for upper extremities than the same amount of conventional rehabilitation, for all types of stroke.
Conclusion
In summary, the majority of studies involving stroke rehabilitation technologies state that they may have positive effects on UE function in stroke patients, but additional studies are needed with greater sample sizes and more precise treatment prescriptions. Specifics are needed to identify how much and how long to utilize the technologies to make the greatest significant differences in UE recovery. In addition, the majority of studies do not utilize patients in their first six months post-stroke. Typically, the greatest neurological recovery is seen in the first three-to-six months following a stroke, which would be the ideal time to investigate the effects of conventional versus technological interventions.
In conclusion, review of the studies mentioned with the use of these technologies, including virtual reality, do not show any negative effects; they only contribute to the patient’s motor recovery and function of UE hemiparesis and would overall complement stroke rehabilitation. RM
Megan Palmer, OTR/L, is a Level III inpatient occupational therapist for Gaylord Specialty Healthcare in Wallingford, Conn. Palmer received her Bachelor of Rehabilitation and Disabilities Studies with a minor in Psychology and her Master’s Degree in Occupational Therapy from Springfield College and has been working with patients with traumatic brain injury and stroke for 15 years.
References
1 Guggisberg AG, Koch PJ, Hummel FC, Buetefisch CM. Brain networks and their relevance for stroke rehabilitation. Clin Neurophysiol. 2019;130(7):1098-1124. doi: 10.1016/j.clinph.2019.04.004
2 Crofts A, Kelly ME, Gibson CL. Imaging Functional Recovery Following Ischemic Stroke: Clinical and Preclinical fMRI Studies. J Neuroimaging. 2020;30(1):5-14. doi: 10.1111/jon.12668
3 Gurbuz N, Afsar SI, Ayaş S, Cosar SN. Effect of mirror therapy on upper extremity motor function in stroke patients: a randomized controlled trial. J Phys Ther Sci. 2016;28(9):2501-2506. doi: 10.1589/jpts.28.2501
4 Green, M. (Aug 2021). PESI, Inc. Stroke Rehab Master Class – Best Practices for Improved Outcomes. Igniting Neuroplasticity after Stroke: Breakthroughs for Improving Motor Recovery.
5 Ward NS, Brander F, Kelly K. Intensive upper limb neurorehabilitation in chronic stroke: outcomes from the Queen Square programme. Journal of Neurology, Neurosurgery & Psychiatry. 2019;90(5):498-506.
6 Knutson JS, Fu MJ, Sheffler LR, Chae J. Neuromuscular Electrical Stimulation for Motor Restoration in Hemiplegia. Phys Med Rehabil Clin N Am. 2015;26(4):729-745. doi: 10.1016/j.pmr.2015.06.002
7 Takeda K, Tanino G, Miyasaka H. Review of devices used in neuromuscular electrical stimulation for stroke rehabilitation. Med Devices (Auckl). 2017;10:207-213. Published 2017 Aug 24. doi: 10.2147/MDER.S123464
8 Wasielewski D. 2010, April. Myomo Robotic Therapy Device. StrokeNetwork Newsletter. Retrieved from Stroke Network: http://strokenetwork.org/newsletter/therapies/myomo.htm
9 Demaitre E. (2019, June 7). Myomo scales up production, training for MyoPro device for upper-body mobility.
10 Peters HT, Page SJ, Persch A. Giving Them a Hand: Wearing a Myoelectric Elbow-Wrist-Hand Orthosis Reduces Upper Extremity Impairment in Chronic Stroke. Arch Phys Med Rehabil. 2017;98(9):1821-1827. doi: 10.1016/j.apmr.2016.12.016
11 MediTouch. (2018). MediTouch HandTutor. Retrieved from MediTouch Homepage: https://meditouch.co.il/products/handtutor/
12 Ikbali Afsar S, Mirzayev I, Umit Yemisci O, Cosar Saracgil SN. Virtual Reality in Upper Extremity Rehabilitation of Stroke Patients: A Randomized Controlled Trial. J Stroke Cerebrovasc Dis. 2018;27(12):3473-3478. doi: 10.1016/j.jstrokecerebrovasdis.2018.08.007
13 Kim JH. Effects of a virtual reality video game exercise program on upper extremity function and daily living activities in stroke patients. J Phys Ther Sci. 2018;30(12):1408-1411. doi: 10.1589/jpts.30.1408
14 Kiper P, Szczudlik A, Agostini M, et al. Virtual Reality for Upper Limb Rehabilitation in Subacute and Chronic Stroke: A Randomized Controlled Trial. Arch Phys Med Rehabil. 2018;99(5):834-842.e4. doi: 10.1016/j.apmr.2018.01.023
[WEB] Improved Motor, Sensory, and Cognitive Recovery of Hand and Arm Function After Stroke
Posted by Kostas Pantremenos in Cognitive Rehabilitation, Paretic Hand, Rehabilitation robotics on January 14, 2022
Posted by Deborah Overman

It happens that, after lying for a while in a way that puts pressure on a nerve in your arm, you do not feel the arm anymore, you cannot perceive its location and size, and it feels like it does not belong to your own body. If this condition lasts for years, the representation of the upper limb in the brain is chronically distorted.
Named body representation disorder, this neurological disorder is one of the more prominent long-term consequences of stroke. It severely affects how people use their body in the environment to move, act, and sense.
Stroke patients report a wide range of symptoms, like being unable to embody their own arm. They also report symptoms like being unable to control the muscles in their arms and hands, being unable to finely modulate grasp force while holding an object, and difficulty in perceiving their arms and hands in general.
If left untreated, sensory and body representation deficits may lead patients to perceive the affected limb as shorter, less sensitive, less responsive, and eventually even to “forget” it.
In the EU, stroke is the leading cause of adult disability according to a 2020 study, and Covid has worsened the scenario. The number of patients with stroke requiring long term assistance and rehabilitation has dramatically increased since the outbreak of COVID-19, as well as flu-related strokes in young people. While some stroke survivors will recover, impairment of the upper limbs can become chronic and seriously affect the behaviur of the patient in up to 75% of stroke patients.
NEUROMUSCULAR ELECTRICAL STIMULATION FOR IMPROVED REHABILITATION
Now, a consortium of neuroscientists, clinicians and neuroengineers, involving the Laboratory of Cognitive Neuroscience of the EPFL (directed by Olaf Blanke), MySpace Lab at CHUV, (directed by Andrea Serino), Villa Beretta Hospital (directed by Franco Molteni), led by the EPFL Translational Neural Engineering laboratory (directed by Silvestro Micera), has shown that carefully tuned electrical stimulation of the neuromuscular system, combined with current rehabilitation practices, are promising for recovering upper limb control and embodiment in stroke patients with long-term disabilities.
The details of their neuromuscular electrical stimulation (NMES) protocols tested on 45 chronic stroke patients are published in MED, the new clinical and translational journal of the CELL editorial family. The project has been funded by CARIGEST and the CARIPLO foundation.
“Our approach has the potential to facilitate neurorehabilitative interventions that target multiple perceptual domains, including tactile acuity, perceived body size, distorted feelings of the arm, and consequently, restored use of the arm.
“Our approach reduced the perceptual dissociation of the affected limb, that’s why it’s so important to pursue targeted electrical stimulation of the muscles in chronic stroke survivors, and to personalize the treatment to counter specific deficits.”
— first author Andrea Crema
Motor and somatosensory improvements persisted after the end of the treatment. Moreover, the electrostimulation protocol improved body representation, i.e. the perceived dimensions and altered feeling towards the affected limb. “Interestingly, reduction of altered feelings correlated with motor improvements, and depended on the quantity of electrostimulation,” Crema adds.
ROBOTIC GLOVE REHAB VS NOVEL NMES REHAB
Forty-five chronic stroke patients underwent 27 sessions of NMES over a period of 9 weeks. Each session lasted 90 minutes, of which 60 consisted of conventional physiotherapy rehabilitation and 30 minutes of a supplemental treatment based on a robotic glove or on custom NMES. The patients were split into three groups with a different mix of conventional rehabilitation and NMES treatment.
The first group used a robotic glove throughout all of the supplemental sessions to perform task-driven exercises. The second group used a novel NMES paradigm in all of the supplemental sessions. The third group used the robotic glove for half of the sessions and the NMES for the other half of the session.
The scientists then measured motor performances, sensory capabilities, and body perception for each patient, before, during, and after the 9-week clinical trial.
Patient performance improved earlier with NMES than with the robotic glove. At the end of the treatment, the motor improvement was higher in groups having partial or complete NMES compared to the glove alone. Also, the improvement extended to somatosensory function and body representation measures.
The current study focuses on chronic stroke patients who had received multiple interventions before, reaching what was considered a plateau of improvement. These results show that targeted, intense intervention, especially via NMES, can push recovery further. They also suggest that but sub-acute stroke patients, those who have just suffered a stroke, may also benefit from NMES, although this has yet to be tested.
“The challenge with sub-acute stroke patients relies in the more volatile sensory perception and body representation. They may have higher benefits from NMES if properly personalised to their quickly changing conditions,” Crema notes.
One of the big novelties of the study is to target and assess not only motor recovery, but also sensory deficits, and body representations.
“This study shows the importance of a multi-faceted assessment of functions after stroke and pave the way to more effective clinical rehabilitation protocols.”
— Silvestro Micera
The scientists are currently working on a new system able to provide finer levels of motor and sensory stimulation, and with broader varieties of stimulation.
[Source(s): Ecole Polytechnique Fédérale de Lausanne, EurekAlert]
[WEB] Improvement in motor, sensory, cognitive recovery of hand, arm function after stroke: Study
Posted by Kostas Pantremenos in Cognitive Rehabilitation, Paretic Hand on January 11, 2022

A new study has found that due to the COVID-19 pandemic, the number of stroke patients requiring long-term assistance and rehabilitation has dramatically increased since the outbreak.
The research has been published in the ‘Cell Journal’.
While some stroke survivors will recover, impairment of the upper limbs can become chronic and seriously affect the behaviour of the patient in upto 75 per cent of stroke patients.
Now, a consortium of neuroscientists, clinicians, and neuroengineers, involving the Laboratory of Cognitive Neuroscience of the EPFL (directed by Olaf Blanke), MySpace Lab at CHUV, (directed by Andrea Serino), Villa Beretta Hospital (directed by Franco Molteni), led by the EPFL Translational Neural Engineering laboratory (directed by Silvestro Micera), has shown that carefully tuned electrical stimulation of the neuromuscular system, combined with current rehabilitation practices are promising for recovering upper limb control and embodiment in stroke patients with long-term disabilities.
“Our approach has the potential to facilitate neurorehabilitative interventions that target multiple perceptual domains, including tactile acuity, perceived body size, distorted feelings of the arm, and consequently, restored use of the arm,” explained the first author Andrea Crema.
He continued, “Our approach reduced the perceptual dissociation of the affected limb, that’s why it’s so important to pursue targeted electrical stimulation of the muscles in chronic stroke survivors and to personalize the treatment to counter specific deficits.”
Motor and somatosensory improvements persisted after the end of the treatment. Moreover, the electrostimulation protocol improved body representation, i.e. the perceived dimensions and altered feeling towards the affected limb.
“Interestingly, reduction of altered feelings correlated with motor improvements, and depended on the quantity of electrostimulation,” explained Crema.
45 chronic stroke patients underwent twenty-seven sessions of NMES over a period of nine weeks. Each session lasted 90 minutes, of which 60 consisted of conventional physiotherapy rehabilitation, and 30 minutes of a supplemental treatment based on a robotic glove or on custom NMES.
The patients were split into three groups with a different mix of conventional rehabilitation and NMES treatment. The first group used a robotic glove throughout all of the supplemental sessions to perform task-driven exercises. The second group used a novel NMES paradigm in all of the supplemental sessions. The third group used the robotic glove for half of the sessions and the NMES for the other half of the session.
The scientists then measured motor performances, sensory capabilities, and body perception for each patient, before, during, and after the nine-week clinical trial.
Patient performance improved earlier with NMES than with the robotic glove. At the end of the treatment, the motor improvement was higher in groups having partial or complete NMES compared to the glove alone. Also, the improvement extended to somatosensory function and body representation measures.
The current study focused on chronic stroke patients who had received multiple interventions before, reaching what was considered a plateau of improvement. These results showed that targeted, intense intervention, especially via NMES, can push recovery further. They also suggested that but sub-acute stroke patients, those who have just suffered a stroke, may also benefit from NMES, although this has yet to be tested.
“The challenge with sub-acute stroke patients relies on the more volatile sensory perception and body representation. They may have higher benefits from NMES if properly personalised to their quickly changing conditions,” explained Crema.
One of the big novelties of the study is to target and assess not only motor recovery, but also sensory deficits, and body representations.
“This study shows the importance of a multi-faceted assessment of functions after stroke and paving the way to more effective clinical rehabilitation protocols,” said Silvestro Micera.
The scientists are currently working on a new system able to provide finer levels of motor and sensory stimulation and with broader varieties of stimulation. (ANI)
[ARTICLE] Neuromuscular Electrical Stimulation improves Activities of Daily Living Post-Stroke: A Systematic Review and Meta-analysis – Full Text
Posted by Kostas Pantremenos in REHABILITATION on November 13, 2021
Abstract
Objectives
1) To elucidate the effectiveness of Neuromuscular Electrical Stimulation (NMES) towards improving activities of daily living (ADL) and functional motor ability post-stroke. 2) Investigate the influence of paresis severity and the timing of treatment initiation for the effectiveness of NMES.
Data Sources
PubMed, MEDLINE, EMBASE, Physiotherapy Evidence Database (PEDro) and Cochrane Library searched for relevant articles from database inception to May 2020.
Study Selection
The inclusion criteria were randomized controlled trials exploring the effect of NMES towards improving ADL or functional motor ability in stroke survivors. The search identified 6064 potential articles with 20 being included.
Data Extraction
Two independent reviewers conducted the data extraction. Methodological quality was assessed using the PEDro scale and the Cochrane Risk of Bias Tool.
Data Synthesis
Data from 428 and 659 participants (mean age 62.4 years; 54% male) for outcomes of ADL and functional motor ability, respectively, were pooled in a random effect meta-analysis. The analysis revealed a significant positive effect of NMES towards ADL (standardized mean difference [SMD] 0.41, 95% confidence interval [CI], 0.14-0.67, P=0.003), whereas no effect on functional motor ability was evident. Subgroup analyses showed that application of NMES in the subacute stage (SMD 0.44, 95% CI 0.09-0.78, P=0.01) and in the upper-extremity (SMD 0.34, 95% CI 0.04-0.64, P=0.02) improved ADL, whereas a beneficial effect was observed for functional motor abilities in severely paretic patients (SMD 0.41, 95% CI 0.12-0.70, P=0.005).
Conclusions
The results of the present meta-analysis are indicative of potential beneficial effects of NMES towards improving ADL post-stroke, whereas the potential for improving functional motor ability appears less clear. Furthermore, subgroup analyses indicated that NMES application in the subacute stage and targeted at the upper-extremity is efficacious for ADL rehabilitation and that functional motor abilities can be positively impacted in severely paretic patients.
——————————–
The global incidence of stroke is in the order of 13.7 million annually1 and is a clinical condition typically associated with limb paresis2 secondary to compromised function of upper motor neurons and associated neural pathways, with loss of locomotor function and the ability to perform activities of daily living (ADL) being functional manifestations hereof.3-5 Although recent medico-scientific advances within the fields of thrombolysis6,7 and thrombectomy8 have spurred major changes to the treatment of acute ischemic stroke, stroke remains a leading cause of disability1 and effective rehabilitation modalities are thus of utmost importance.
In the newest Clinical Guidelines for Stroke Management9 and Guidelines for Adult Stroke Rehabilitation and Recovery,10 the rehabilitation modality of ‘Electrical Stimulation’ (ES) is recommended as a supplementary therapy alongside the standard care modalities. ES can be broadly categorized into functional electrical stimulation (FES) and therapeutic electrical stimulation (TES). The primary difference between these two ES modalities is the degree of patient involvement, as TES is administered with the patient completely passive or performing isolated muscle contractions, whereas FES is superimposed onto voluntary contractions while the patient is performing functional tasks such as walking, rising from a chair, or stair climbing.11,12 As alluded by Kroon et al.13 TES can be further sub-categorized into Neuromuscular electrical stimulation (NMES), EMG-triggered electrical stimulation (EMG-ES), positional feedback stimulation training (PFST) and transcutaneous electrical nerve stimulation (TENS). In EMG-triggered and positional feedback stimulation, the electrical current is administered in response to the patient performing a minor contraction or movement, respectively, whereas NMES is administered according to a pre-programmed scheme and is hence received passively.11,14,15 Evidence suggests that NMES has the ability to strengthen muscles,16,17 reduce spasticity,10 increase excitability of corticospinal neural pathways18 and augment neuroplasticity.19,20 Furthermore, when electrical stimulation is administered prior to or following voluntary contractions (e.g. NMES) in healthy subjects, it has been demonstrated to be more effective in developing functional motor abilities than both voluntary contractions performed simultaneously with stimulation and voluntary contractions performed in isolation.20,21 The apparent superiority could be governed by a cumulative effect of the two types of contractions and/or due to the unique motor drives associated with each type of contraction.21,22
According to the International Classification of Functioning, Disability and Health (ICF), post-stroke rehabilitation is a complex process that can be viewed in the context of function, activity and participation domains.23 The activity domain encompasses the full range of life areas from a performance and capacity point of view, where the performance level describes an individual’s abilities in the actual context in which they live (ADL) and the capacity level entails the ability to execute a specific task or action in a standard environment (functional motor ability).23 ADL reflects the level of disability in daily life and is therefore thought of as the most clinically relevant outcome in assessing post-stroke recovery,24 whereas functional motor abilities are viewed as good surrogate outcomes.
A number of systematic reviews concerning the effectiveness of ES towards regaining overall activity performance post-stroke have been published, including three Cochrane reviews.17,25,26 However, the majority of said reviews have pooled studies with a variety of ES methods16,25,27-31 or investigated other specific aspects of ES, typically FES.24,32-34 In contrast to previous systematic reviews, the aim of the present systematic review and meta-analysis was to elucidate the effectiveness of NMES in improving ADL and functional motor ability post-stroke and additionally analyze data according to onset of NMES administration post-stroke and paresis severity, which in our opinion are important additions to the stroke rehabilitation literature.[…]
[ARTICLE] Feasibility and preliminary efficacy of a combined virtual reality, robotics and electrical stimulation intervention in upper extremity stroke rehabilitation – Full Text
Posted by Kostas Pantremenos in Tele/Home Rehabilitation, Virtual reality rehabilitation on April 19, 2021
Abstract
Background
Approximately 80% of individuals with chronic stroke present with long lasting upper extremity (UE) impairments. We designed the perSonalized UPper Extremity Rehabilitation (SUPER) intervention, which combines robotics, virtual reality activities, and neuromuscular electrical stimulation (NMES). The objectives of our study were to determine the feasibility and the preliminary efficacy of the SUPER intervention in individuals with moderate/severe stroke.
Methods
Stroke participants (n = 28) received a 4-week intervention (3 × per week), tailored to their functional level. The functional integrity of the corticospinal tract was assessed using the Predict Recovery Potential algorithm, involving measurements of motor evoked potentials and manual muscle testing. Those with low potential for hand recovery (shoulder group; n = 18) received a robotic-rehabilitation intervention focusing on elbow and shoulder movements only. Those with a good potential for hand recovery (hand group; n = 10) received EMG-triggered NMES, in addition to robot therapy. The primary outcomes were the Fugl-Meyer UE assessment and the ABILHAND assessment. Secondary outcomes included the Motor Activity Log and the Stroke Impact Scale.
Results
Eighteen participants (64%), in either the hand or the shoulder group, showed changes in the Fugl-Meyer UE or in the ABILHAND assessment superior to the minimal clinically important difference.
Conclusions
This indicates that our personalized approach is feasible and may be beneficial in improving UE function in individuals with moderate to severe impairments due to stroke.
Introduction
Approximately 80% of individuals with stroke experience hemiparesis of the upper extremity (UE) [1] leading to chronic impairments such as weakness, loss of motor control, edema, pain and spasticity. These have important consequences for quality of life as impairments in hand and arm function limit participation in activities of daily living [2, 3]. Accordingly, recovery of UE function is seen as highly important by individuals with chronic stroke, caregivers and rehabilitation professionals [4].
According to the Canadian Stroke Best Practices [5], UE rehabilitation should involve the affected limb in “training that is meaningful, engaging, repetitive, progressively adapted, task-specific and goal-oriented”. Advances in rehabilitation technology, in particular robotics, virtual reality (VR) and neuromuscular electrical stimulation (NMES), have been shown to be individually effective for improving UE function of individuals with stroke, through the provision of such repetitive and task-oriented training. Robotic devices can be used to assist individuals who are unable to complete arm movements by themselves [6]. Robotic rehabilitation has demonstrated functional gains in individuals with mild and moderate stroke impairments [7,8,9]. Likewise, some of our recent work [10] has shown that individuals with severe, chronic stroke can improve their arm range of motion and clinical scores after ten sessions of robotic therapy. However, it should be noted that functional gains in robotic therapy are not greater than those obtained with similar intensity conventional therapy [8]. While the intensity of practice is a determining factor in stroke recovery, higher improvements might have been achieved by robotic therapy if its focus was not only on shoulder and elbow movements, but also on hand function. This may be possible by integrating robotic therapy in a rehabilitation program that also includes other modalities that better target hand function.
VR activities constitute another approach to UE stroke rehabilitation, where patients typically perform movements without physical assistance. Reviews examining the use of VR for the improvement of UE function show promising results [11, 12]. In our view, VR could consolidate the UE functional gains obtained through robotic rehabilitation. While most VR activities typically focus on shoulder and elbow movements, some recent technical advances now allow the inclusion of hand movements as well. Specifically, the Microsoft Kinect version 2, used to track movements in VR, can detect hand opening and closing in addition to shoulder, elbow and wrist movements. These capabilities have been included in a new rehabilitation application, targeting UE reaching and grasping movements [13], which was part of our rehabilitation approach.
Electromyographically (EMG)-triggered NMES is a muscle stimulation modality that has been used to facilitate motor recovery of the hand after stroke [14]. The individual with stroke needs to activate the muscle(s) volitionally to trigger the NMES [15]. Thus, EMG-triggered NMES provides wrist and/or finger extension time-locked to the cognitive movement intent to actively extend the wrist and open the hand, making the training ecological and functionally relevant. EMG-triggered NMES has been shown to improve voluntary activation of isolated muscles, particularly in task-specific patterns [16].
While advances in robotics, VR and NMES have led to new treatment modalities targeting UE function post-stroke, further progress is needed for these technologies to have a true impact. Despite numerous studies attempting to identify the most effective rehabilitation interventions, post-stroke UE recovery remains disappointing [17] with sensorimotor deficits persisting in a large proportion of stroke survivors for more than 6 months (up to 62% [18]). Improvements in clinical scores have been small and often fail to meet the criteria for minimal clinically important differences (MCID) [19]. While most of the recent clinical trials have failed to demonstrate improvements on UE function that favour new interventions such as robotics or VR, over conventional, dose-matched therapy [20], combination of different modalities may have a greater impact on stroke recovery than each individual modality alone [21]. Thus, there is a need to look beyond the ‘one-size-fits-all’ approach, where a single UE modality is applied to a group of post-stroke individuals. Another possible reason for the relatively small gains in UE function, and in particular the low gains in hand function [17], is that an individual’s potential for recovery is not always considered [20]. In clinical practice, therapists typically prescribe UE exercises to their clients based on initial clinical measures, which turn out to be poor predictors of future UE function [22]. However, assessing the integrity of the affected corticospinal tract (CST), by means of motor evoked potentials (MEPs) elicited by non-invasive transcranial magnetic stimulation (TMS), was found to strongly predict the changes in UE function that could be elicited by rehabilitation [23]. In particular, the work by Milot et al. [24] showed that amongst several brain measures (e.g., magnetic resonance imaging, diffusion tensor imaging), baseline MEP amplitude was the best predictor of the response to robotic training of the affected UE in chronic stroke survivors. The presence of a MEP indicates that the CST, linking the motor areas of the brain to the hand musculature, is at least partially preserved.
Considering that (1) an individualized intervention to post stroke UE rehabilitation is desirable, (2) CST integrity is a strong predictor of hand function recovery, and (3) combination of different modalities may have a greater impact on stroke recovery than each individual modality alone, our proposed approach was to combine multiple modalities in an individualized intervention, tailored to each stroke participant’s functional status and recovery potential. Recovery may be enhanced by first assessing CST integrity in order to determine the potential for recuperating hand function, and then combining multiple purposefully selected combinations of modalities to target motor deficits of each individual. Specifically, our perSonalized UPper Extremity Rehabilitation (SUPER) program included: (1) robotic activities to work on physically assisted UE reaching movements; (2) VR activities to work on unassisted reaching and grasping movements; and (3) NMES to facilitate hand opening and closing movements. The frequency of incorporation of each modality during the intervention was determined according to the individual’s potential for hand recovery. Our objectives were to determine the feasibility and the treatment effect of the SUPER program in individuals with moderate/severe chronic stroke. Our hypotheses were that (1) the SUPER program would be feasible in terms of process, resources, management and safety indicators and (2) stroke participants with a low potential for hand recovery would benefit from a shoulder/elbow-centered intervention, while those with a high potential would benefit from an intervention involving the whole arm.[…]
