Posts Tagged spinal cord injury
[PhD Thesis] ADDRESSING THE GAPS IN ASSESSMENT AND TREATMENT OF THE UPPER EXTREMITY IN REHABILITATION OF INDIVIDUALS WITH NEUROLOGICAL CONDITIONS – Full Text PDF
Posted by Kostas Pantremenos in Functional Electrical Stimulation (FES), Paretic Hand, REHABILITATION on December 4, 2021
Regaining upper extremity function is critical following stroke and spinal cord injury
(SCI). The objectives of this thesis were to develop and assess the psychometric
properties of a 3D printed version of an upper extremity outcome assessment tool called the Toronto Rehabilitation
Institute-Hand Function Test (TRI-HFT) and to assess the feasibility of stimulating interscapular
muscles using transcutaneous functional electrical stimulation (FES).
The first study explored the feasibility of 3D printing the original TRI-HFT objects and
assessed its inter and intra-rater reliability and convergent validity in chronic stroke. The second
study focused on assessing its psychometric properties in the sub-acute and chronic SCI
populations. We hypothesized that the TRI-HFT could be 3D printed and that the 3D printed test
would have high reliability and validity in stroke and SCI populations. In the third study I
explored the feasibility and benefits of stimulating the Lower Trapezius (LT), Serratus Anterior
(SA) and Upper Trapezius (UT) along with Anterior Deltoid during forward flexion and along
with Middle Deltoid during abduction in able-bodied individuals. The underlying hypothesis was
that it would result in an increased range and more natural reaching movement.
In the first and second study we found that all objects of the TRI-HFT could be
successfully 3D printed with an error margin of less than 10% except for the Paper and the
Sponge objects. The 3D TRI-HFT showed high inter and intra-rater reliability in stroke and SCI.
The 3D TRI-HFT showed strong criterion validity when compared to the Graded Redefined
Assessment of Strength, Sensibility and Prehension test in the SCI population. The 3D TRIHFT showed moderate to strong construct validity when compared to the Chedoke-McMaster
Stroke Assessment-Arm and Hand and the Fugl Meyer Assessment-Hand in chronic stroke. In
the third study, the LT, SA and UT could be successfully stimulated using surface FES. The
maximum reach in abduction for FES of middle deltoid along with the interscapular muscles
was 51.77°±17.54° compared to FES for middle deltoid alone which was 43.76°±15.32°.
This work essentially builds on the current state of assessment and FES treatment of the
upper extremity in the rehabilitation domain.[…]
[Abstract] Robotic assistive and rehabilitation devices leading to motor recovery in upper limb: a systematic review
Posted by Kostas Pantremenos in Paretic Hand, Rehabilitation robotics on April 18, 2021
Abstract
Purpose
Stroke, spinal cord injury and other neuromuscular disorders lead to impairments in the human body. Upper limb impairments, especially hand impairments affect activities of daily living (ADL) and reduce the quality of life. The purpose of this review is to compare and evaluate the available robotic rehabilitation and assistive devices that can lead to motor recovery or maintain the current motor functional level.
Methods
A systematic review was conducted of the literature published in the years from 2016–2021, to focus on the most recent rehabilitation and assistive devices available in the market or research environments.
Results
A total of 230 studies published between 2016 and 2021 were identified from various databases. 107 were excluded with various reasons. Twenty-eight studies were taken into detailed review, to determine the efficacy of robotic devices in improving upper limb impairments or maintaining the current level from getting worse.
Conclusion
It was concluded that with a good strategy and treatment plan; appropriate and regular use of these robotic rehabilitation and assistive devices do lead to improvements in current conditions of most of the subjects and prolonged use may lead to motor recovery.
- Implications for Rehabilitation
- Stroke, accidents, spinal cord injuries and other neuromuscular disorders lead to impairments. Upper limb impairments have a tremendous adverse affect on ADL and reduces quality of life drastically.
- Advancement in technology has led to the designing of many robotic assistive and rehabilitation devices to assist in motor recovery or aid in ADL.
- This review analyses different available devices for rehabilitation and assistance and points out that use of these devices in time does help in motor recovery. Most of the studies reviewed showed improvements for the user.
- Future devices should be more portable and easier to use from home,
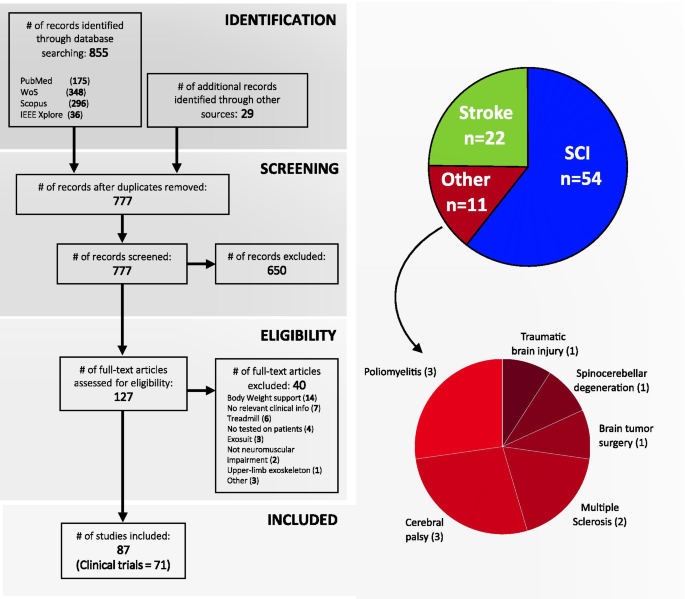
[ARTICLE] Systematic review on wearable lower-limb exoskeletons for gait training in neuromuscular impairments – Full Text
Posted by Kostas Pantremenos in Gait Rehabilitation - Foot Drop, REHABILITATION, Rehabilitation robotics on March 4, 2021
Abstract
Gait disorders can reduce the quality of life for people with neuromuscular impairments. Therefore, walking recovery is one of the main priorities for counteracting sedentary lifestyle, reducing secondary health conditions and restoring legged mobility. At present, wearable powered lower-limb exoskeletons are emerging as a revolutionary technology for robotic gait rehabilitation. This systematic review provides a comprehensive overview on wearable lower-limb exoskeletons for people with neuromuscular impairments, addressing the following three questions: (1) what is the current technological status of wearable lower-limb exoskeletons for gait rehabilitation?, (2) what is the methodology used in the clinical validations of wearable lower-limb exoskeletons?, and (3) what are the benefits and current evidence on clinical efficacy of wearable lower-limb exoskeletons? We analyzed 87 clinical studies focusing on both device technology (e.g., actuators, sensors, structure) and clinical aspects (e.g., training protocol, outcome measures, patient impairments), and make available the database with all the compiled information. The results of the literature survey reveal that wearable exoskeletons have potential for a number of applications including early rehabilitation, promoting physical exercise, and carrying out daily living activities both at home and the community. Likewise, wearable exoskeletons may improve mobility and independence in non-ambulatory people, and may reduce secondary health conditions related to sedentariness, with all the advantages that this entails. However, the use of this technology is still limited by heavy and bulky devices, which require supervision and the use of walking aids. In addition, evidence supporting their benefits is still limited to short-intervention trials with few participants and diversity among their clinical protocols. Wearable lower-limb exoskeletons for gait rehabilitation are still in their early stages of development and randomized control trials are needed to demonstrate their clinical efficacy.
Background
Gait disorders affect approximately 60% of patients with neuromuscular disorders [1] and generally have a high impact on their quality of life [2]. Moreover, immobility and loss of independence for performing basic activities of daily living results in patients being restricted to a sedentary lifestyle. This lack of physical activity increases the risk of developing secondary health conditions (SHCs), such as respiratory and cardiovascular complications, bowel/bladder dysfunction, obesity, osteoporosis and pressure ulcers [3,4,5,6,7]; which can further reduce the patients’ life expectancy [3, 4]. Therefore, walking recovery is one of the main rehabilitation goals for patients with neuromuscular impairments [8, 9].
Robotic gait rehabilitation appeared 25 years ago as an alternative to conventional manual gait training. Compared with conventional therapy, robotic gait rehabilitation can deliver highly controlled, repetitive and intensive training in an engaging environment [10], reduce the physical burden for the therapist, and provide objective and quantitative assessments of the patients’ progression [11]. The use of gait rehabilitation robots began in 1994 [12] with the development of Lokomat [13]. Since then, different rehabilitation robots have been developed and can be classified into grounded exoskeletons (e.g., Lokomat [14], LOPES [15], ALEX [16]), end-effector devices (e.g., Gait Trainer [17], Haptic Walker [18]), and wearable exoskeletons (e.g., ReWalk [19], Ekso [20], Indego [21]) [12]. In addition, there have been recent developments towards “soft exoskeletons” or “exosuits” which use soft actuation systems and/or structures to assist the walking function [22,23,24,25]. Despite these developments, to date the optimal type of rehabilitation robot for a specific user and neuromuscular impairment still remains unclear [26].
Wearable exoskeletons are emerging as revolutionary devices for gait rehabilitation due to both the active participation required from the user, which promotes physical activity [27], and the possibility of being used as an assistive device in the community. The number of studies on wearable exoskeletons during the past 10 years has seen a rapid increase, following the general tendency now towards rehabilitation robots [28]. Some of these devices already have FDA approval and/or CE mark, and are commercially available, whereas many others are still under development.
There have been several reviews surveying the field of wearable exoskeletons for gait rehabilitation. Some of these reviews have focused on reviewing the technological aspects of exoskeletons from a general perspective [29, 30], while others have focused on specific aspects such as the control strategies [31] or the design of specific joints [32]. A selection of reviews have focused on surveying the evidence on effectiveness and usability of exoskeletons for clinical neurorehabilitation in general [33, 34], or for a specific pathology such as spinal cord injury (SCI) [30, 34] or stroke [11].
This review provides a comprehensive overview on wearable lower-limb powered exoskeletons for over ground training, without body weight support, that are intended for use with people who have gait disorders due to neuromuscular impairments. In comparison with other reviews, we analyse a wide range of aspects of wearable exoskeletons, from their technology to their clinical evidence, for different types of pathologies. This systematic review was carried out to address the following questions: (1) what is the current technological status of wearable lower-limb exoskeletons for gait rehabilitation?, (2) what are the benefits and risks for exoskeleton users?, and (3) what is the current evidence on clinical efficacy for wearable exoskeletons?
[WEB PAGE] Getting REAL About Functional Therapy
Posted by Kostas Pantremenos in REHABILITATION on February 8, 2021
By Tracie Hunnicutt, MS, CCC-SLP; Julie Clement, OTR; and Susan Adix, PT
Patients enter inpatient rehabilitation facilities for countless reasons, but always share a common goal—independence. Each patient may define independence differently, but the ultimate goal for all is to recover function in the skill sets that allow them to return to their chosen activities at home or in the community. The foundation for successful recovery of function is task specificity. Research has shown that physical rehabilitative therapy focused on task-specific training produces more meaningful functional improvements than therapy based on high-intensity repetitive exercise alone.1 This is true regardless of age or diagnosis, and is particularly relevant for patients who are recovering from a neurologic event. When the neurophysiologic goal is to impact plasticity, it is critical that the activities chosen for therapy are the very same activities that patients will be returning to at home or in the community. Task-specific practice results in greater cortical representation and reorganization in recovering neuro patients.1 In patient populations other than neuro, task specificity may not be necessary to impact plasticity, but can and will incrementally improve both patient performance and confidence.
Functional therapy begins with a skilled therapist invested in developing an individualized care plan suited to the patient’s needs and interests. The joint nature of the care plan should start during a thorough patient history, where both skill and intuition are necessary to identify what is important to the patient, and what therapy tasks can most closely resemble the patient’s desired activities. Physical therapists, occupational therapists, and speech language pathologists all spend a large portion of their work day adjusting tasks or manipulating the surrounding environment to simulate a patient’s home and community activities. This can present a challenge during the inpatient portion of a patient’s recovery in both acute care and inpatient rehabilitation settings, which are hospital-based and designed with patient safety and ease of function as priorities. The contrast between what the environment demands from a patient in a hospital versus the demands of home and community is vast, and can be a surprise and a risk to patients who are not prepared.
From this clinical need arose a new treatment tool—Realistic Environment Applied Learning, or REAL Therapy. REAL Therapy is a community simulation environment with ready-made functional therapy tasks available to patients and clinicians from the moment they enter the room. Because the environment is task-specific, and does not require modification by the therapist, the entire time spent in therapy can address the physical and cognitive demands of real-life activities performed by the patient. REAL Therapy is comprised of various modules that each have a unique clinical focus. Modules were designed to address some of the most common community-based locations patients visit upon discharge. Available modules include a grocery store, restaurant, deli, laundromat, and a car transfer/gas station area.

Grocery Store
The grocery store module is the most versatile and commonly used area of REAL Therapy. Opportunities for functional therapy tasks abound, and physical therapists, occupational therapists, and speech pathologists alike utilize this module daily because of the variety of tasks that can be performed. The grocery store includes standard grocery shelving with a height of 72 inches, stocked with realistically weighted items. There is an area with fruit and vegetables that must be hand selected and weighed, as well as a bakery. A freezer with cold storage contains commonly refrigerated items. There are shopping carts and baskets, a working checkout counter, a register, and an ATM.
Some of the common physical tasks include dynamic balance activities such as pushing a shopping cart, carrying a shopping basket, reaching for objects on high shelves, reaching for objects on low shelves, reaching for objects at the back of shelves, opening the glass door and selecting items from cold storage, picking up heavy or bulky objects, retrieving items from a shopping cart and placing them on the checkout counter, bagging items, and carrying bags out of the store. Some of the common cognitive tasks include creating and executing a shopping list, locating difficult-to-find items, reading labels, calculating totals, money management, operating a credit card machine, staying within a budget, using memory strategies to recall short lists of items, and identifying obstacles and safety hazards.
This module is used frequently because it represents an essential community location that up to 84% of geriatric adults visit regularly.2 Community-based locations specific to food and medical care are among the most commonly visited sites for older adults. Therefore, task-specific practice with a skilled therapist is likely to be a precursor for greater success and safety when patients are functioning in these locations post-discharge.
Restaurant
The restaurant is another module with a variety of applications depending on the patient’s individual needs or preferences. There are various settings within the restaurant, each with its own challenges to the patient. There is indoor booth-style seating for one table and outdoor seating/patio furniture for another, complete with a table umbrella.
Physical tasks for this module include getting into and out of a booth with limited space, pulling out chairs to sit down and repositioning closer to the table once seated, raising or lowering the table umbrella, reaching across the table to receive food from a server, and identifying a space to place any necessary assistive devices (ie, walkers, wheelchairs, canes, etc) while at the table. Common cognitive or communication tasks for this module include reading a menu, making menu choices that consider dietary restrictions or special needs, communicating with wait staff, verbalizing an order, participating in conversation and socialization during a meal, estimating a bill, calculating a tip, completing and signing the check, and time management.

Deli
The deli module includes a glass-faced display case from which patients can view and select their food options. There is a metal tray line in front of the display case followed by a drink and condiment station. This module demands more from the patients from a mobility standpoint, and is less flexible in terms of the ability to modify the physical tasks that can be performed.
Common physical tasks for the deli include standing and reaching into the display case to obtain food, placing items on the tray, pushing the tray down the line as it gains an increasing amount of weight, moving the tray to the drink station, obtaining the desired drink and condiments, carrying the tray, and getting into and out of seating. The deli has an elevated barstool-type seating area, and the restaurant module seating may also be used. Where the deli lacks some flexibility in physical modification of tasks, it is particularly useful in the patient’s ability to problem-solve in a challenging, less forgiving environment.
Cognitively, the patient must determine if he or she has the ability to perform the necessary tasks, or if it would be safer to request help with the physical components of this setting. Elements of the deli environment, such as bilateral upper extremity use to open the case and obtain the food or propelling and carrying the tray, may require the assistance of another person, as they cannot be easily modified. Cognitive tasks in the deli include visual scanning, sequencing food choices (ie, salad, main dish, dessert, drink), making food choices that are compliant with dietary needs, estimating cost, money management, and identification of barriers or safety hazards.
Laundromat
The laundry module can represent a community-based laundromat or a home-based laundry room. This module includes a top-loading washer, a front-loading dryer, an ironing board, and an elevated folding table that also has a place for hanging clothes. Common physical tasks in the laundromat include picking up large piles of both wet and dry clothing, picking up heavy containers of detergent or fabric softener, loading and removing clothing from the washer, bending to load and remove clothing from the dryer, carrying a laundry basket, folding clothes, ironing clothes, and hanging clothes. Common cognitive tasks in this module include sorting and organizing clothing, calculating how much detergent or fabric softener to use, and time management. If this module is being used to simulate an actual laundromat, there is an available change machine to include the money management portion of the activity.

Car Transfer / Gas Station
In addition to the grocery store, the car transfer/gas station module is one of the more frequently utilized areas of REAL Therapy. Safe and effective car transfers are often one of the keys to a patient’s continued community involvement, as well as access to necessities and medical care following hospitalization. The inability to perform this task has been linked to decreased quality of life, increased burden of care, and the possibility of institutional living.3 In certain patient populations such as spinal cord injury, correct execution of car transfers is even more critical to prevent pain and injury that could compromise a patient’s overall independence.4 The REAL Therapy module features a car transfer simulator. The simulator is the front end of a car, with working doors, handles, locks, seat belts, bench or bucket seats, gas pedal, brake, steering wheel, and a behind-seat wheelchair loading area. The car simulator is height adjustable, so that therapists may match the simulator to the type of car, truck, or SUV utilized by each patient.
When utilizing the car simulator, patients must perform a variety of physical tasks that include approaching the vehicle, opening the car door, getting into proper position for the transfer, turning and lowering themselves into the car seat, bringing their legs into the vehicle, repositioning in the seat if necessary, and buckling the seat belt. One of the more challenging aspects of car transfers is the management of assistive devices during the transfer. Patients who are learning to get in and out of a vehicle often need and depend upon assistive devices, but find that there is limited space between the car door and seat. They require instruction from a skilled therapist for proper placement and utilization of devices such as wheelchairs, power wheelchairs, walkers, hemi-walkers, canes, sliding boards, crutches, etc. Another aspect of managing those assistive devices is ensuring that the caregiver or family member is trained and able to lift and/or stow the devices in the car. The car simulator has behind-seat space specifically designed for practicing this skill. Caregiver training for proper body mechanics will help prevent both injury and broken equipment.
The simulator also has the ability to assess the driver’s brake reaction speed with a reaction time tester. Drivers are given instructions to attend to an illuminated light box with red and green lights. As the green light changes to red, the timer starts and the patient depresses the brake. Reaction speeds are generated and may be compared to age and gender norms as a means of basic biofeedback to the patient. Information from various studies has revealed that in some patient populations, reaction time is a useful metric in determining when it is appropriate to return to driving.5 While this decision is ultimately made jointly by the physician and patient, the data generated by the reaction time tester can be valuable information and a means to build insight and confidence for patients and families.
Other elements included in the car transfer/gas station module are an ADA-compliant flooring surface that simulates asphalt, a 6-inch curb typical of those found in parking lots, and a weighted gas pump.
While the benefit of REAL Therapy for patients is clear, the group of people who may be the most invested in REAL Therapy are the rehabilitation clinicians. The amount of time they spend trying to modify the environment to suit the needs of each patient can become direct patient care time. There are also endless possibilities of treatment ideas, so there is less time spent planning and organizing, and more time spent doing. REAL Therapy also removes the necessity of explaining to the patient how the therapy tasks they are doing are applicable to their real-life activities, as the connection is very clear. Rather than moving weights on a shelf to simulate groceries, the patient walks into a grocery store. Rather than move from one chair to another, the patient gets into a car. This immediately makes sense to the patient, increasing their buy-in and willingness to work with therapy.
REAL Therapy is the manifestation of a treatment philosophy that focuses on returning a patient to function. With the move to shorter lengths of stay across all healthcare settings, it is important that physical rehabilitation therapists immediately focus on the patient’s desired activities and begin task-specific training as early as possible. In addition, the evidence base supports functional, task-specific training to achieve better patient outcomes and perceived independence. When used in conjunction with a protocol for therapeutic patient outings into the community, there is an even larger impact on patient confidence and ability. As the rehabilitation industry and we as therapy professionals move forward, REAL Therapy and functional, task-specific treatment may be the key to efficient service delivery and excellent patient outcomes. RM
Tracie Hunnicutt, MS, CCC-SLP, Therapy Manager, received her training as a speech-language pathologist at Texas Tech University Health Sciences Center. The early part of her career was focused on the care of traumatic brain injury (TBI) patients with an emphasis on community re-entry. From this setting, she developed a strong foundation in functional therapy as a tool to return patients back to school, work, community, and home activities. Hunnicutt is currently a medical speech language pathologist in inpatient rehabilitation at HealthSouth Rehabilitation Hospital of Arlington.
Julie Clement, OTR, received her training in occupational therapy at the University of Texas Medical Branch. She specializes in helping patients increase independence with Activities of Daily Living (ADLs), functional mobility, and Instrumental Activities of Daily Living (IADLs). Clement is a Neuro-IFRAH certified therapist, and has completed more than 200 hours of continuing education related to neurological evaluation and treatment. She has been the OT Team Lead at HealthSouth Rehabilitation Hospital of Arlington for the past 12 years.
Susan Adix, PT, received her training as a physical therapist at the University at Buffalo. She began her career in outpatient physical therapy at HealthSouth Arlington, working primarily with orthopedic and neurologic patient populations. In 2006, Adix transitioned into inpatient rehabilitation, where she focused her professional development on the care of neurologic patients. Adix has assisted in the development of the REAL Therapy Gym and is currently the PT Team Lead for a staff of more than 30 physical therapists and rehabilitation techs. For more information, contact RehabEditor@medqor.com.
References
1. Bayona NA, Bitensky J, Salter K, Teasell R. The role of task-specific training in rehabilitation therapies. Top Stroke Rehabil. 2005;12(3):58-65.
2. Brown C, Bradberry C, Howze S, Hickman L, Ray H, Peel C. Defining community ambulation from the perspective of the older adult. J Geriatr Phys Ther. 2010;33:56-53.
3. Elrod C, Bass B, Colvin K. Identification of the key components of car transfers by individuals with dementia. J Geriatr Phys Ther. 2005;28(3):122-123.
4. Haubert LL, Mulroy SJ, Hatchett PE, et al. Car transfer and wheelchair loading techniques in independent drivers with paraplegia. Front Bioeng Biotechnol. 2015 Sep 17;3:139.
5. Dickerson A. Standardizing the RT-2S brake reaction time tester. NewsBrake. 2010;Winter:22-25.
[ARTICLE] Activity-based training with the Myosuit: a safety and feasibility study across diverse gait disorders – Full Text
Posted by Kostas Pantremenos in Gait Rehabilitation - Foot Drop, REHABILITATION, Rehabilitation robotics on November 30, 2020
Abstract
Background
Physical activity is a recommended part of treatment for numerous neurological and neuromuscular disorders. Yet, many individuals with limited mobility are not able to meet the recommended activity levels. Lightweight, wearable robots like the Myosuit promise to facilitate functional ambulation and thereby physical activity. However, there is limited evidence of the safety and feasibility of training with such devices.
Methods
Twelve participants with diverse motor disorders and the ability to walk for at least 10 m were enrolled in this uncontrolled case series study. The study protocol included five training sessions with a net training time of 45 min each. Primary outcomes were the feasibility of engaging in training with the Myosuit, the occurrence of adverse events, and participant retention. As secondary outcomes, we analyzed the walking speed using the 10-m Walk Test (10MWT) and for three participants, walking endurance using the 2-min Walk Tests.
Results
Eight out of 12 participants completed the entire study protocol. Three participants withdrew from the study or were excluded for reasons unrelated to the study. One participant withdrew because of an unsafe feeling when walking with the Myosuit. No adverse events occurred during the study period for any of the participants and all scheduled trainings were completed. For five out of the eight participants that completed the full study, the walking speed when using the Myosuit was higher than to their baseline walking speed.
Conclusions
Activity-based training with the Myosuit appears to be safe, feasible, and well-tolerated by individuals with diverse motor disorders.
Background
Physical inactivity has been identified as the fourth leading risk factor for global mortality, only surpassed by hypertension, tobacco use, and hyperglycemia. To contain the risks associated with physical inactivity, the World Health Organization recommends that all adults engage in moderate intensity physical activity for at least 150 min each week [1].
Physical activity is also a recommended part of treatment for stroke patients [2], and for patients with incomplete spinal cord injury (SCI) [3], inherited neuropathies such Charcot–Marie–Tooth disease [4], heart failure [5], or chronic obstructive pulmonary disease [6]. These wide-ranging recommendations reflect the consistent association between increased physical activity and improved health-related quality of life (e.g. [7,8,9].).
In spite of the evident health benefits of physical activity, a large proportion of elderly individuals and individuals with limited mobility do not meet the recommended dose of physical activity in their daily lives [10]. In many of these cases, neurological, neuromuscular, or cardiovascular deficits prevent individuals from reaching moderate intensity levels during exercise. In some cases, they prohibit any voluntary exercise altogether.
To address this problem, various technological solutions like full-leg, rigid exoskeletons have been developed to assist overground mobility (e.g. [11,12,13,14].). The safety and feasibility of gait training with mobile exoskeletons has been evaluated in several longitudinal training studies for individuals with spinal cord injury [15,16,17] and hemiparesis following stroke [18, 19]. Rigid exoskeletons largely substitute the ambulatory function of severely affected or completely paralyzed individuals and enable them to walk. Electric motors are used to provide large assistive torques to the users’ leg joints via rigid linkages. This allows exoskeletons to support the majority of the users’ weight and advance the users’ legs without a major voluntary contribution from the leg muscles.
The typically large masses of mobile rigid exoskeletons increase limb inertia and thereby hinder walking at higher speeds. The highest walking speed achieved in previous training studies [15,16,17,18,19] was 0.67 m/s, while most speeds were as low as 0.1 m/s to 0.4 m/s. This is well below the speeds required to support individuals with residual mobility during moderate intensity exercise.
To assist this more capable section of the population, more lightweight wearable robots (also known as “exosuits”, “exomuscles” or “dermoskeletons”) have been proposed [20,21,22,23,24]. Unlike exoskeletons that act on all leg joints, these devices allow for—and require—the active participation of the user, and can (partially) assist walking over a larger range of speeds [20, 25]. Thereby, such wearable robots can provide assistance as needed for functional ambulation [25] while simultaneously modulating the cardiovascular load of their users according to exercise recommendations. For example, a soft robotic exosuit unilaterally acting on one ankle joint was shown to reduce the energy expenditure and interlimb asymmetry of individuals with hemiparesis following a stroke during walking at 0.5 to 1.3 m/s [20]. In previous work from our group, we demonstrated that a soft wearable robot actively supporting hip and knee extension, the Myosuit, enabled an individual with incomplete SCI to walk substantially faster when assisted [25]. More recently, we showed that this functional improvement also translates to an increase in exercise intensity and a momentary reduction of the energetic cost of transport [26]. In larger longitudinal studies, training with wearable robots acting on the hip joint was shown to result in an intrinsic reduction of the cost of transport for elderly individuals [23] and individuals following stroke [24].
Further work [27,28,29] has primarily focused on the functional effects of robotic movement assistance. A lightweight wearable robot that assists knee flexion and extension reduced momentary movement performance, but elicited larger intrinsic improvements after 2 weeks of exercise training than when training without the device in users with multiple sclerosis [27]. In larger randomized controlled trials with individuals post-stroke, training with a robotic knee brace was shown to result in only modest functional benefits that were comparable to the control group [28], while training with a hip exoskeleton resulted in more pronounced functional benefits [29].
While it is hard to synthesize common trends out of the limited studies available, it appears that the most pronounced improvements were so far achieved with devices that targeted a very specific motor deficit (e.g. ankle assistance [20] or hip assistance [24, 29] improved hemiparetic gait for individuals after stroke). Other findings with devices that bear promise to work for more diverse gait disorders were based on single-participant observations [22, 25, 26], and it remains unclear to what extend these results generalize to larger populations.
There is limited evidence of how a single wearable robot could effectively assist individuals with diverse neuromuscular and neurological gait disorders during exercise training. Such a wider applicability would be highly desirable considering that in everyday clinical life, patients present with a wide array of different conditions and functional deficits [30].
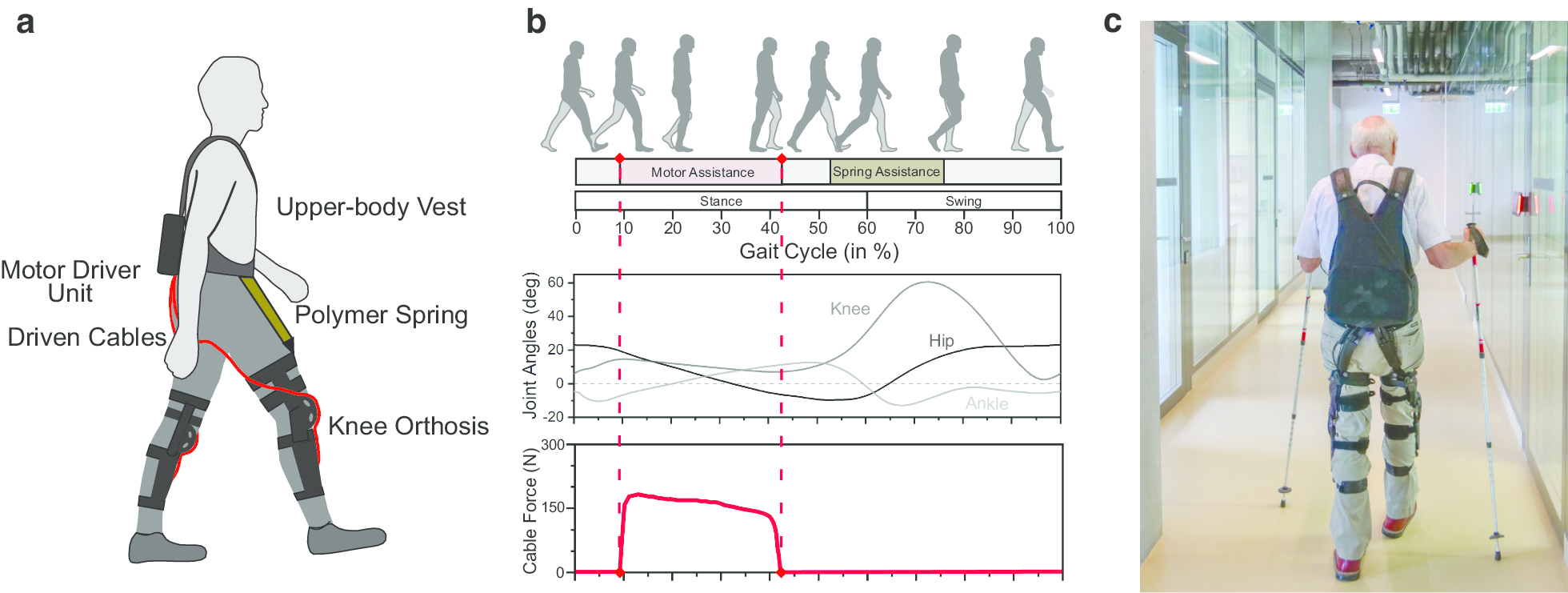
We believe that the Myosuit is a promising device to assist the training of individuals with diverse gait disorders. The Myosuit assists walking in essential functions [31] by supporting the user’s bodyweight from weight acceptance through mid-stance and assisting swing initiation from terminal stance into early swing (see Fig. 1b). By working in parallel with the user’s hip and knee extensor muscles and exploiting natural synergies [32], the Myosuit supports the muscles with the largest contribution to bodyweight support in that phase of walking [33]. Training with the Myosuit can extend beyond step training and into areas of balance, strength, and coordination.
[WEB PAGE] Aquatic Occupational Therapy
Posted by Kostas Pantremenos in REHABILITATION on November 14, 2020
Posted by Debbie Overman

by Lauren Pocius, OTR/L, and Leigh Riley, MSOT
Aquatic therapy continues to grow in popularity all over the country in many rehabilitation settings. It is mainly used as a safe addition and/or alternative to land-based therapy due to the benefits of the water. At Gaylord Specialty Healthcare in Wallingford, Conn, aquatic occupational therapy is used on an inpatient and outpatient basis to improve patients’ overall daily functioning and quality of life. The focus of this article is to describe the benefits and goals of aquatic occupational therapy and the complementary role it plays in patient care. A case study will be used to further explain and discuss the benefits of aquatic occupational therapy intervention.
Facility Overview
Gaylord Specialty Healthcare in Wallingford functions as a long-term acute care hospital and outpatient rehabilitation facility. Individuals receiving medical treatment and therapeutic intervention at this facility are often referred for specialized care dealing with acute and chronic illness or injury. Gaylord is the only hospital in Connecticut, and one of two facilities in the country, with Commission on Accreditation of Rehabilitation Facilities (CARF) international accreditation for all inpatient rehabilitation programs, as well as additional accreditation for spinal cord injury, stroke, and brain injury. Gaylord Hospital aims to provide the most comprehensive and cutting-edge care for its patient population. The inpatient and outpatient therapy programs at Gaylord offer access to state-of-the-art equipment and treatment interventions to best meet rehabilitation needs. The Gaylord outpatient clinic in Wallingford treats a wide range of orthopedic and neurologic diagnoses, including stroke, spinal cord injury, traumatic brain injury, orthopedic injuries, hip and knee replacements, multiple sclerosis, and pulmonary disease.
Interventions Occupational Therapists Provide
Occupational therapy practitioners construct occupation-based interventions to facilitate functional change and growth for their clients. This process involves clinical evaluation, intervention, and targeting of outcomes. The process requires a collaborative relationship between the therapist and client.1 Keeping in mind the goal of improved engagement in meaningful occupation, occupational therapy practitioners focus on interventions that include the maintenance or improvement of joint and muscle active range of motion, flexibility, balance, fine and gross motor coordination, muscular and cardiovascular strength and endurance, sensory reintegration and re-education, and functional cognitive skills.
Goals established in occupational therapy may appear to be similar throughout the client population with respect to functional independence. However, the chosen interventions and activities used in treatment sessions can be vastly different due to the individualized path each client takes toward improved life engagement.
Aquatic Therapy Program: Occupational Therapy
The outpatient occupational therapy aquatic program was created this year to offer the most comprehensive treatment options for those transitioning from inpatient services, as well as for those being referred from other facilities. The aquatics program at Gaylord has been in place since 1987. Before the current therapeutic pool was built in 1992, patients would receive aquatic intervention at local fitness centers.
Gaylord Hospital’s aquatic center has a large 75-foot by 25-foot pool that is maintained at a constant 90 degrees. The pool is designed with specialized lifts, steps with handrails, and a 2-foot-high ledge that allows individuals of all functional mobility levels to enter the pool with ease. The pool includes a submerged bench, handhold cut-outs, a deep-water exercise station for modified pull-ups and push-ups, and warm-water jets located throughout the sides of the pool. Additional pool equipment includes step benches, buoyancy cuffs and belts, Velcro weights, webbed gloves, cervical collars, noodle floats, paddles, barbells, and dumbbells of varied thicknesses. The pool’s design and various aquatic equipment aim to address balance, strengthening, endurance, pain management, muscle relaxation, and modified functional task completion. The aquatic center also offers a program called Aquacize, which is an inclusive pool exercise program for all ages and ability levels.
Aquatic therapy offers a unique set of benefits for practitioner and client to maximize rehabilitation. The viscosity and buoyancy of water provides support for the client and assists in completion of balance tasks with improved safety and confidence. The properties of water in a therapeutic setting can facilitate decreased joint/soft-tissue edema, which can assist with pain management. Warm water temperature increases joint flexibility and range of motion, promotes relaxation, and improves circulation. Water’s natural viscosity and resistance can be utilized for muscle strengthening and endurance. Aquatic therapy also provides a zero-gravity environment for those with weight-bearing limitations. By reducing the load on joints during movement, exercises in water are often performed with improved range and tolerance. This zero-gravity environment also provides the opportunity for a treating therapist to position a client in the water in a way that is not safe or practical for those with mobility impairments on land.
The aquatic setting provides a less intimidating environment for practice of simulated functional tasks such as object carrying, stepping, item retrieval, and placement. There are instances for individuals receiving land-based therapy where these skills are unsafe or inappropriate to practice on land. Practicing a functional task in water assists in the progression toward safe and confident land-based activity for an individual receiving therapy. Aquatic therapeutic intervention is just as individualized as land-based treatment and relates to all aspects of the occupational therapy framework.
Goals of Occupational Therapy Aquatics
The ultimate goal is to maximize functional independence and ease in daily task completion, as well as transition the individual to an independent exercise program. For example, by improving upper extremity active range of motion, balance, and coordination, a patient will be able to reach up into their cabinets while cooking a meal more easily. With increased muscle strength and endurance, a patient can improve their safety while getting themselves dressed. Patients will also improve their overall quality of life with reduced pain levels. For certain diagnoses, including nerve injuries, the water can improve their body awareness and sensation due to sensory reintegration and neuromuscular re-education.
Case Study: Tim
Pmhx:
Tim is a 52-year-old male who was in good health when he fell off a forklift at work May 24, 2018, and experienced an incomplete C4 spinal cord injury as a result.
Tim had several surgeries following his injuries and was placed on a ventilator in an attempt to stabilize his condition for maximal recovery. Postoperatively, Tim began to regain some movement and sensation to his left leg. His recovery continued after being transferred to Gaylord Specialty Healthcare for inpatient rehabilitation. Tim’s recovery was remarkable, as he began therapy as a bedbound patient and walked out of the hospital with only a rollator on the day of his discharge.
Despite his improvements, Tim continues to experience increased muscle spasticity, which limits his upper extremity range of motion and functional use, as well as decreased strength, endurance, coordination, dexterity and gait disturbance.
Evaluation/Treatment on Land
Tim began outpatient occupational therapy in October 2018, 5 months following his injury. Initially, it was observed that Tim was doing very well despite his impairments. Therapy sessions began focusing on improving his upper extremity range of motion, functional use, and decreasing muscle spasticity. Activities of daily living (ADLs) were also addressed to improve Tim’s quality of life. Tim has made great progress during his time in outpatient, as he is now living independently in his own apartment. However, progress with Tim’s upper extremity function has been slow and began to plateau over the past several months. Tim has done well with learning how to compensate for his deficits and figuring out how to manage all of life’s challenges with about half the normal range of motion in his upper extremities.
Benefits of Aquatics
Due to his lack of progress, Tim and his therapists agreed that aquatic occupational therapy would be a great complement to his land-based intervention and would assist in his progress.
The focus of pool therapy sessions for Tim involved using the properties of water in a therapeutic environment to improve upon his bilateral upper extremity passive range of motion, active range of motion, tone management, and sensory reintegration.
Upon entering the water for the first time, Tim immediately commented on the weightlessness of his body. Since his injury, Tim has felt a constant pressure and strain on his joints. The buoyancy created in an aquatic environment reduced the load on Tim’s joints, allowing his body to relax in a way he had not been able to experience on land. This assists in decreasing muscle spasticity to allow for improved range of motion. Tim’s therapy sessions have focused on using the Bad Ragaz Method along with prolonged stretch of his upper extremities in the water to assist in improvement in active and passive range of motion.
The Bad Ragaz Method involves the therapist positioning an individual horizontally in water using the support of flotation devices. The therapist moves the patient through the water, creating resistance with the goal of promoting muscle relaxation, pain management, and improved neuromuscular functioning.2 Post therapy sessions, Tim reported reduced pain, improvement in active range of motion of his arms, and longer periods of decreased spasticity than with land-based intervention alone.
Functional Gains
Improvements in foundational skills such as range of motion, strength, coordination, and sensation, are areas that allow for overall improvement in independence and life quality. Outside of the physical benefits observed, Tim also reports that being in the water allows his body to move in ways he is unable to on land, which is an exciting and meaningful experience for him. Occupational therapy at its core aims to provide therapeutic intervention through meaningful occupations that are individualized from person to person. This aquatic environment not only assists in Tim’s overall function but has meaning to him personally and his rehabilitation journey.
Patients with both similar and different diagnoses to Tim can benefit from aquatic occupational therapy in an outpatient setting. For example, a patient with multiple sclerosis can benefit from aquatics due to water buoyancy for decreased strain on joints while improving their overall strength and endurance. A patient who has suffered multiple rib fractures in a motor vehicle accident can improve their upper extremity range of motion and decrease pain in water in a way he would be unable to on land. The properties of water can have a positive effect on almost any diagnosis, which is why aquatic therapy continues to grow in popularity.
Conclusion
Occupational therapy strives to improve patients’ function with their daily tasks through meaningful and purposeful interventions. Aquatic occupational therapy is a creative approach to rehabilitation that allows patients to maximize function in a completely different environment. The benefits of aquatic rehab are immense and can improve a patient’s overall quality of life. In Tim’s case, he continues to see improvements both in the water and on land. Aquatic occupational therapy will continue to provide patients with new meaningful and purposeful interventions and be an enjoyable addition in their recovery. RM
Lauren Pocius, OTR/L, is an occupational therapist at Gaylord Specialty Healthcare, Wallingford, Conn.
Leigh Riley, MSOT, is an occupational therapist at Gaylord Specialty Healthcare, Wallingford, Conn. For more information, contact RehabEditor@medqor.com.
References
1. Occupational Therapy Practice Framework: Domain and Process (3rd Edition). (2014). Am J Occup Ther. 68(Supplement_1). doi:10.5014/ajot.2014.68s1
2. Hands-On Aquatic Therapy Techniques for the Ortho/Neuro Client: A Survey Course (2014). Aquatic Therapy University
[ARTICLE] Functional Electrical Stimulation Therapy for Retraining Reaching and Grasping After Spinal Cord Injury and Stroke – Full Text
Posted by Kostas Pantremenos in Functional Electrical Stimulation (FES), Paretic Hand on November 3, 2020
Neurological conditions like hemiplegia following stroke or tetraplegia following spinal cord injury, result in a massive compromise in motor function. Each of the two conditions can leave individuals dependent on caregivers for the rest of their lives. Once medically stable, rehabilitation is the main stay of treatment. This article will address rehabilitation of upper extremity function. It is long known that moving the affected limb is crucial to recovery following any kind of injury. Overtime, it has also been established that just moving the affected extremities does not suffice, and that the movements have to involve patient’s participation, be as close to physiologic movements as possible, and should ideally stimulate the entire neuromuscular circuitry involved in producing the desired movement. For over four decades now, functional electrical stimulation (FES) is being used to either replace or retrain function. The FES therapy discussed in this article has been used to retrain upper extremity function for over 15 years. Published data of pilot studies and randomized control trials show that FES therapy produces significant changes in arm and hand function. There are specific principles of the FES therapy as applied in our studies: (i) stimulation is applied using surface stimulation electrodes, (ii) there is minimum to virtually no pain during application, (iii) each session lasts no more than 45–60 min, (iv) the technology is quite robust and can make up for specificity to a certain extent, and (v) fine motor function like two finger precision grip can be trained (i.e., thumb and index finger tip to tip pinch). The FES therapy protocols can be successfully applied to individuals with paralysis resulting from stroke or spinal cord injury.
Introduction
Application of functional electrical stimulation (FES) for therapeutic purposes in rehabilitation settings dates back to the 1960’s when Liberson et al. (1961) used an FES system to stimulate the peroneal nerve to correct foot drop by triggering a foot switch, a single-channel electrical stimulation device stimulated the common peroneal nerve via a surface electrode, producing ankle dorsiflexion during the swing phase of gait (Liberson et al., 1961). This led to the first commercially available FES system with surface stimulation electrodes. Since then FES technology has been researched extensively to evaluate its benefits in diverse neurological conditions, and using an array of application techniques (Baldi et al., 1998; Field-Fote, 2001; Popovic et al., 2005, 2011, 2012, 2016; Yan et al., 2005; Frotzler et al., 2008; Griffin et al., 2009; Daly et al., 2011; Kapadia et al., 2011, 2013, 2014a; Giangregorio et al., 2012; Malešević et al., 2012; Martin et al., 2012; Kawashima et al., 2013; Lee et al., 2013; Sadowsky et al., 2013; Ho et al., 2014; Kapadia N. et al., 2014; Popović, 2014; Sharif et al., 2014; Bauer et al., 2015; Howlett et al., 2015; Vafadar et al., 2015; Buick et al., 2016; Cuesta-Gómez et al., 2017; Fu et al., 2019; Straudi et al., 2020). The two common uses of FES are to replace function (i.e., as an orthotic device) and to retrain function (i.e., as a therapeutic device). In this article we will limit ourselves to the therapeutic application of FES.
In the therapeutic application (FES therapy), FES is used as a short-term treatment modality. The expectation is that, after training with the FES system, the patients will be able to voluntarily perform the trained activities without FES (i.e., patients are expected to regain voluntary function). To date, a few high-quality randomized controlled trials have been performed, proving the efficacy of FES therapy over other rehabilitation techniques (Sharififar et al., 2018; Yen et al., 2019). This paucity in multicenter randomized controlled trials and the limited access to systems that can properly deliver FES therapy might have affected its uptake in clinical settings (Ho et al., 2014; Auchstaetter et al., 2016). Fortunately, both these issues are being addressed as new FES systems that are specifically developed for FES therapy are being introduced, as well as large scale multicenter randomized controlled trials are being planned to further confirm the efficacy of this rehabilitation modality. This article will provide readers with the details on how transcutaneous multichannel FES therapy for the upper extremity can be applied in clinical trials and as such the same methodology can be used in clinical practice by physiotherapists and occupational therapists.
The FES methodology discussed here has been developed with the intent to be user friendly, robust and to be able to produce better functional gains than the presently available best-practice rehabilitation techniques. The FES system used in our laboratory is a surface stimulation system with up to 4 stimulation channels that can produce gross motor function as well as precision grips such as two finger pinch grip. However, the methodology of FES application discussed here is pertinent to any multichannel transcutaneous FES device. We have used transcutaneous FES to retrain reaching and grasping in individuals with both spinal cord injury and stroke (Thrasher et al., 2008; Kapadia and Popovic, 2011; Kapadia et al., 2011, 2013; Popovic et al., 2012; Hebert et al., 2017). The results obtained in both patient populations indicate functional improvements after 8–14 weeks of therapy (20–48 h of stimulation). Patients showed reduced dependency on caregivers, and some even became independent in their activities of daily living.
This article will extensively detail how FES was applied in these previously successful clinical trials to retrain reaching and grasping functions in individuals who sustained a spinal cord injury or a stroke.[…]
[Abstract] Applications of Head-Mounted Displays for Virtual Reality in Adult Physical Rehabilitation: A Scoping Review – Review
Posted by Kostas Pantremenos in REHABILITATION, Virtual reality rehabilitation on October 17, 2020
Abstract
Importance: Head-mounted displays for virtual reality (HMD–VR) may be used as a therapeutic medium in physical rehabilitation because of their ability to immerse patients in safe, controlled, and engaging virtual worlds.
Objective: To explore how HMD–VR has been used in adult physical rehabilitation.
Data Sources: A systematic search of MEDLINE, Embase, Cochrane Library, CINAHL, Web of Science, PsycINFO, and ERIC produced 11,453 abstracts, of which 777 underwent full-text review.
Study Selection and Data Collection: This scoping review includes 21 experimental studies that reported an assessment or intervention using HMD–VR in a physical rehabilitation context and within the scope of occupational therapy practice.
Findings: HMD–VR was used for assessment and intervention for patients with a range of disorders, including stroke, multiple sclerosis, spinal cord injury, and Parkinson’s disease.
Conclusions and Relevance: HMD–VR is an emerging technology with many uses in adult physical rehabilitation. Higher quality clinical implementation studies are needed to examine effects on patient outcomes.
What This Article Adds: We review existing research on how immersive virtual reality (e.g., using head-mounted displays) has been used for different clinical populations in adult physical rehabilitation and highlight emerging opportunities in this field for occupational therapists.
[ARTICLE] Functional electrical stimulation therapy for restoration of motor function after spinal cord injury and stroke: a review – Full Text
Posted by Kostas Pantremenos in Functional Electrical Stimulation (FES), Neuroplasticity, REHABILITATION on September 28, 2020
Abstract
Functional electrical stimulation is a technique to produce functional movements after paralysis. Electrical discharges are applied to a person’s muscles making them contract in a sequence that allows performing tasks such as grasping a key, holding a toothbrush, standing, and walking. The technology was developed in the sixties, during which initial clinical use started, emphasizing its potential as an assistive device. Since then, functional electrical stimulation has evolved into an important therapeutic intervention that clinicians can use to help individuals who have had a stroke or a spinal cord injury regain their ability to stand, walk, reach, and grasp. With an expected growth in the aging population, it is likely that this technology will undergo important changes to increase its efficacy as well as its widespread adoption. We present here a series of functional electrical stimulation systems to illustrate the fundamentals of the technology and its applications. Most of the concepts continue to be in use today by modern day devices. A brief description of the potential future of the technology is presented, including its integration with brain–computer interfaces and wearable (garment) technology.
Background
Losing the ability to move voluntarily can have devastating consequences for the independence and quality of life of a person. Stroke and spinal cord injury (SCI) are two important causes of paralysis which affect thousands of individuals around the world. Extraordinary efforts have been made in an attempt to mitigate the effects of paralysis. In recent years, rehabilitation of voluntary movement has been enriched by the constant integration of new neurophysiological knowledge about the mechanisms behind motor function recovery. One central concept that has improved neurorehabilitation significantly is neuroplasticity, the ability of the central nervous system to reorganize itself during the acquisition, retention, and consolidation of motor skills [1]. In this document, we present one of the interventions that has flourished as a consequence of our increased understanding of the plasticity of the nervous system: functional electrical stimulation therapy or FEST. The document, which is not a systematic review, is intended to describe early work that played an important historical role in the development of this field, while providing a general understanding of the technology and applications that continue to be used today. Readers interested in systematic reviews of functional electrical simulation (FES) are directed to other sources (e.g., [2,3,4]).[…]
Continue
[Abstract] Lessons learned from robotic gait training during rehabilitation: Therapeutic and medical severity considerations over 3 years
Posted by Kostas Pantremenos in Gait Rehabilitation - Foot Drop, REHABILITATION, Rehabilitation robotics on September 21, 2020
Abstract
BACKGROUND: Robotic exoskeletons are increasingly available to inpatient rehabilitation facilities though programmatic implementation evidence is limited.
OBJECTIVE: To describe therapists’ clinical practice experiences with robotic gait training (RGT) over 3 years during inpatient rehabilitation.
METHODS: Therapists participated in a survey and semi-structured focus group to discuss their RGT experiences. Interviews were recorded, transcribed, and analyzed using a theoretical analysis-driven thematic approach.
RESULTS: Therapists averaged 7.6 years of neurologic rehabilitation experience and 1.85 years with RGT. Eight of 10 therapists had completed ⩾ 50 RGT sessions, with frequency of 1–5 sessions/week, including on-label (spinal cord injury, stroke) and off-label (e.g., traumatic brain injury) experiences. Three adverse events occurred over 716 RGT sessions with 186 patients. Qualitative analysis identified three major themes and corresponding subthemes: 1-Comparison with traditional gait training approaches (6 sub-themes), 2-Clinical decision-making considerations (3), and 3-On-label and off-label utilization (4). Stated RGT benefits included decreased therapists’ physical burden and increased patient motivation. Clinical concerns with RGT included tonicity, continence, and patient communication (e.g., aphasia). Off-label RGT was used to overcome barriers in traditional gait therapy and achieve early mobility.
CONCLUSIONS: Therapists’ level of training and clinical knowledge furthered RGT implementation and allowed for safe utilization with on-label and off-label patients.
Source: https://content.iospress.com/articles/technology-and-disability/tad190248
