Posts Tagged Stroke
[Abstract] Evaluation of custom-made VR exergame for at-home Stroke rehabilitation. A longitudinal single-arm study. – Full Text PDF
Posted by Kostas Pantremenos in Paretic Hand, Video Games/Exergames, Virtual reality rehabilitation on April 26, 2024
Abstract
Exercise games (Exergames) based on Virtual Reality (VR) have emerged as a promising option for supporting physical rehabilitation in stroke users. As a com- plementary therapy, they offer valuable benefits such as therapy engagement and enjoyment. In this study, we assessed the effectiveness of an immersive, custom- made VR exergame designed for upper limb rehabilitation in stroke participants aged 50 and above. We conducted 14 sessions of 15 minutes involving ten par- ticipants (6 females, ages 58.1 ± 7.5 years old) who volunteered to participate in an assisted at-home rehabilitation process. The study employed a range of evaluation tests to measure physical rehabilitation and game user experience out- comes. The tests included pre- and post-assessments of range of motion (ROM), the Ashworth spasticity test, and the Borg rating of perceived fatigue question- naire. To evaluate the game participant experience, we used the VR Neuroscience Questionnaire (VRNQ), and the Immersive Tendencies Questionnaire (ITQ). Our results revealed significant improvements in the range of motion for elbow and shoulder flexion, extension, adduction, and abduction. Furthermore, we observed a reduction in Ashworth spasticity, and the fatigue scale showed reduced per- ception comparing the last with the first session, although the difference was insignificant. The VRNQ questionnaire indicated significant enhancements in the domains related to ”Game Experience” and ”Game Mechanics” and an overall reduction of the perceived “Motion Sickness”. In the ITQ questionnaire, partic- ipants reported high levels of ”Attention,” and while there were no significant differences in ”Immersion” and ”Enjoyment,” a considerable improvement was observed in ”Excitement”. In summary, our results indicate that the immersive VR exergame improved the range of motion, spasticity, and overall game user experience among participants with stroke in a longitudinal, single-arm inter- vention. We conclude that using custom-made VR exergames is an effective and motivating tool for upper limb rehabilitation, with positive changes in both clin- ical and perception outcomes, and the positive and measurable effects persist after the first sessions. These findings support using VR exergames as a comple- mentary tool for at-home rehabilitation therapy with good ease of use, improved physical rehabilitation outcomes, and high treatment adherence.
[Abstract] Evaluation of custom-made VR exergame for at-home Stroke rehabilitation. A longitudinal single-arm study. – Full Text PDF
Posted by Kostas Pantremenos in Tele/Home Rehabilitation, Video Games/Exergames, Virtual reality rehabilitation on April 21, 2024
Abstract
Exercise games (Exergames) based on Virtual Reality (VR) have emerged as a promising option for supporting physical rehabilitation in stroke users. As a com- plementary therapy, they offer valuable benefits such as therapy engagement and enjoyment. In this study, we assessed the effectiveness of an immersive, custom- made VR exergame designed for upper limb rehabilitation in stroke participants aged 50 and above. We conducted 14 sessions of 15 minutes involving ten par- ticipants (6 females, ages 58.1 ± 7.5 years old) who volunteered to participate in an assisted at-home rehabilitation process. The study employed a range of evaluation tests to measure physical rehabilitation and game user experience out- comes. The tests included pre- and post-assessments of range of motion (ROM), the Ashworth spasticity test, and the Borg rating of perceived fatigue question- naire. To evaluate the game participant experience, we used the VR Neuroscience Questionnaire (VRNQ), and the Immersive Tendencies Questionnaire (ITQ). Our results revealed significant improvements in the range of motion for elbow and shoulder flexion, extension, adduction, and abduction. Furthermore, we observed a reduction in Ashworth spasticity, and the fatigue scale showed reduced per- ception comparing the last with the first session, although the difference was insignificant. The VRNQ questionnaire indicated significant enhancements in the domains related to ”Game Experience” and ”Game Mechanics” and an overall reduction of the perceived “Motion Sickness”. In the ITQ questionnaire, partic- ipants reported high levels of ”Attention,” and while there were no significant differences in ”Immersion” and ”Enjoyment,” a considerable improvement was observed in ”Excitement”. In summary, our results indicate that the immersive VR exergame improved the range of motion, spasticity, and overall game user experience among participants with stroke in a longitudinal, single-arm inter- vention. We conclude that using custom-made VR exergames is an effective and motivating tool for upper limb rehabilitation, with positive changes in both clin- ical and perception outcomes, and the positive and measurable effects persist after the first sessions. These findings support using VR exergames as a comple- mentary tool for at-home rehabilitation therapy with good ease of use, improved physical rehabilitation outcomes, and high treatment adherence.
[Abstract] Impact of telehealth on stroke survivor–caregiver dyad in at-home rehabilitation: A systematic review
Posted by Kostas Pantremenos in Caregivers, Tele/Home Rehabilitation on April 19, 2024
Abstract
Aim
To examine studies involving the impact of telerehabilitation (TLR), tele-training and tele-support on the dyad stroke survivor and caregiver in relation to psychological, physical, social and health dimensions.
Design
A systematic review was conducted.
Data Sources
The following electronic databases were consulted until September 2023: PsycInfo, CINAHL, Eric, Ovid, PubMed, Scopus, Cochrane Central and Web of Science.
Review Methods
It was conducted and reported following the checklists for Reviews of PRISMA 2020 Checklist. Critical evaluation of the quality of the studies included in the review was performed with the Joanna Briggs Institute Checklists.
Data Synthesis
A total of 2290 records were identified after removing duplicates, 501 articles were selected by title and abstract and only 21 met the inclusion criteria. It included 4 quasi-experimental studies, 7 RCTs, 1 cohort study and 9 qualitative studies. The total number of participants between caregivers and stroke survivors was 1697, including 858 stroke survivors and 839 caregivers recruited from 2002 to 2022. For a total of 884 participants who carried out TLR activities in the experimental groups,11 impact domains were identified: cognitive/functional, psychological, caregiver burden, social, general health and self-efficacy, family function, quality of life, healthcare utilization, preparedness, quality of care and relationship with technology.
Conclusions
The results support the application of telehealth in the discharge phase of hospitals and rehabilitation centres for stroke survivors and caregivers. TLR could be considered a substitute for traditional rehabilitation only if it is supported by a tele-learning programme for the caregiver and ongoing technical, computer and health support to satisfy the dyad’s needs.
[ARTICLE] Noninvasive spinal stimulation improves walking in chronic stroke survivors: a proof-of-concept case series – Full Text
Posted by Kostas Pantremenos in Gait Rehabilitation - Foot Drop on April 8, 2024
Abstract
Background
After stroke, restoring safe, independent, and efficient walking is a top rehabilitation priority. However, in nearly 70% of stroke survivors asymmetrical walking patterns and reduced walking speed persist. This case series study aims to investigate the effectiveness of transcutaneous spinal cord stimulation (tSCS) in enhancing walking ability of persons with chronic stroke.
Methods
Eight participants with hemiparesis after a single, chronic stroke were enrolled. Each participant was assigned to either the Stim group (N = 4, gait training + tSCS) or Control group (N = 4, gait training alone). Each participant in the Stim group was matched to a participant in the Control group based on age, time since stroke, and self-selected gait speed. For the Stim group, tSCS was delivered during gait training via electrodes placed on the skin between the spinous processes of C5–C6, T11–T12, and L1–L2. Both groups received 24 sessions of gait training over 8 weeks with a physical therapist providing verbal cueing for improved gait symmetry. Gait speed (measured from 10 m walk test), endurance (measured from 6 min walk test), spatiotemporal gait symmetries (step length and swing time), as well as the neurophysiological outcomes (muscle synergy, resting motor thresholds via spinal motor evoked responses) were collected without tSCS at baseline, completion, and 3 month follow-up.
Results
All four Stim participants sustained spatiotemporal symmetry improvements at the 3 month follow-up (step length: 17.7%, swing time: 10.1%) compared to the Control group (step length: 1.1%, swing time 3.6%). Additionally, 3 of 4 Stim participants showed increased number of muscle synergies and/or lowered resting motor thresholds compared to the Control group.
Conclusions
This study provides promising preliminary evidence that using tSCS as a therapeutic catalyst to gait training may increase the efficacy of gait rehabilitation in individuals with chronic stroke.
Trial registration NCT03714282 (clinicaltrials.gov), registration date: 2018-10-18.
Background
Stroke is the leading cause of adult-onset disability [1]. Despite many advances in gait research in the last decade, about 35% of stroke survivors fail to regain independence in performing activities of daily living due to the impaired function of their affected leg, and about 70% have gait deficits, including reduced walking speeds, asymmetrical walking patterns, and motor coordination issues [2,3,4].
Walking deficits after stroke mostly derive from a disruption of the corticospinal pathways that play an important role in transmitting sensory–motor commands [5, 6]. To address this, most interventions using non-invasive electrical pulses focus on stimulation of the motor cortex to activate dormant or new pathways [2, 7, 8]. However, while supra-spinal regions can facilitate fine locomotor control, spinal networks ultimately generate the basic locomotor pattern [9, 10]. More interestingly, a recent study using functional MRI showed increased blood-oxygen-level dependent activities in motor cortex following transcutaneous spinal cord stimulation (tSCS) in individuals with stroke [11]. Therefore, we hypothesized that tSCS would facilitate an improvement of gait after stroke. Our previous work, in collaboration with additional researchers, established anatomical and physiological changes in the spinal cord after stroke [12, 13], offering a theoretical basis for testing our hypothesis of targeting the spinal circuits for post-stroke recovery.
Recently, Moshonkina et al. reported functional improvements in post-stroke individuals after 2 weeks of tSCS with standard physical therapy, achieving the minimum clinical important differences (MCID) in the 6 min walk test and comfortable walking speed [14]. The same investigators reported immediate improvements in walking kinematics after a single tSCS session [15, 16]. Notably, however, none of the studies mentioned above investigated the effects of more than 4 weeks of training nor tried to explore the potential neurophysiological differences accompanied with gait outcomes. Consequently, it remains unclear whether tSCS can exert a lasting impact on restoration of function following a stroke.
We investigated whether tSCS combined with symmetry-focused gait training has a sustained effect on gait recovery after chronic stroke. We hypothesized that longer-term gait training (24 sessions) with tSCS would lead to greater sustained improvements in walking function compared to control treatment focused solely on gait training. Specifically, we focused on gait symmetry since such improvements can have lasting effects on balance and overall mobility of stroke survivors [6]. We also expected that gait improvements would be associated with physiological changes in muscle coordination measured from electromyography (EMG) of the paretic side, and spinal excitability determined by the spinal motor evoked responses (sMERs). […]
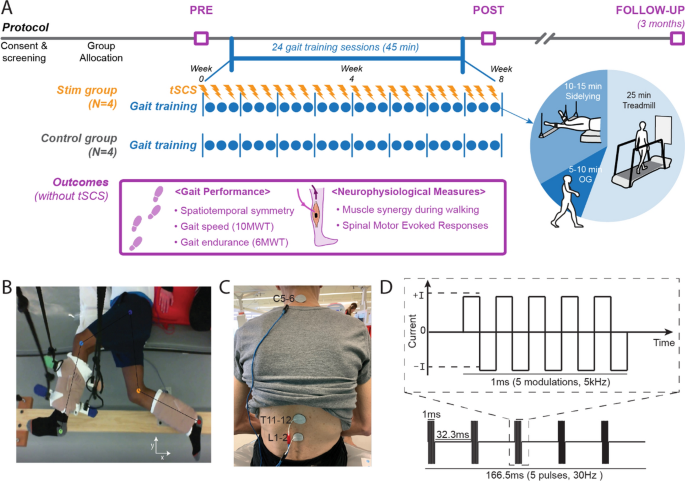
Study protocol and stimulation setup. A Overall experimental protocol. B Top–down view of position of the legs extended beyond the edge of the table and supported with vertically cables during the side-lying training of a participant (Stim 2). C tSCS delivered using surface electrodes on the skin between the C5–6, T11–12, and L1–2 spinous processes (cathode) and a surface electrode on each anterior crest (anode, not shown). D Schematic representation of biphasic pulse sequence used for tSCS. tSCS transcutaneous spinal cord stimulation, OG overground walking, 10MWT 10-m walk test, 6MWT 6-min walk test
[Abstract] Neurostimulation for treatment of post-stroke impairments
Posted by Kostas Pantremenos in Epilepsy on April 8, 2024
Abstract
Neurostimulation, the use of electrical stimulation to modulate the activity of the nervous system, is now commonly used for the treatment of chronic pain, movement disorders and epilepsy. Many neurostimulation techniques have now shown promise for the treatment of physical impairments in people with stroke. In 2021, vagus nerve stimulation was approved by the FDA as an adjunct to intensive rehabilitation therapy for the treatment of chronic upper extremity deficits after ischaemic stroke. In 2024, pharyngeal electrical stimulation was conditionally approved by the UK National Institute for Health and Care Excellence for neurogenic dysphagia in people with stroke who have a tracheostomy. Many other approaches have also been tested in pivotal device trials and a number of approaches are in early-phase study. Typically, neurostimulation techniques aim to increase neuroplasticity in response to training and rehabilitation, although the putative mechanisms of action differ and are not fully understood. Neurostimulation techniques offer a number of practical advantages for use after stroke, such as precise dosing and timing, but can be invasive and costly to implement. This Review focuses on neurostimulation techniques that are now in clinical use or that have reached the stage of pivotal trials and show considerable promise for the treatment of post-stroke impairments.
Key points
- Neurostimulation techniques are ideally suited for use during stroke recovery owing to their ability to target anatomical structures or neuronal networks, alongside precise timing and dosing.
- Paired invasive vagus nerve stimulation has been shown to increase the number of people who achieve clinically important improvements in upper extremity impairment and performance of functional tasks following stroke. The treatment is now in clinical use in the USA.
- Several other neurostimulation techniques show promise for post-stroke impairments but definitive data from adequately powered trials are lacking.
- Pharyngeal electrical stimulation increases the odds of decannulation following tracheostomy and is under investigation as a treatment for post-stroke dysphagia.
[Preprint] Feasibility of Simultaneous Transcranial Direct Current Stimulation During Gait Training in Chronic Stroke Patients: A Randomized Double-blind Pilot Clinical Trial
Posted by Kostas Pantremenos in Gait Rehabilitation - Foot Drop, tDCS/rTMS on April 2, 2024
Abstract
Background
Transcranial direct current stimulation (tDCS) is a therapeutic tool for improving post-stroke gait
disturbances, with ongoing research focusing on specific protocols for its application. We evaluated the
feasibility of a rehabilitation protocol that combines tDCS with conventional gait training.
Methods
This was a randomized, double-blind, single-center pilot clinical trial. Patients with unilateral hemiplegia
due to ischemic stroke were randomly assigned to either the tDCS with gait training group or the sham
stimulation group. The anodal tDCS electrode was placed on the tibialis anterior area of the precentral
gyrus while gait training proceeded. Interventions were administered 3 times weekly for 4 weeks.
Outcome assessments, using the 10-meter walk test, Timed Up and Go test, Berg Balance Scale,
Functional Ambulatory Scale, Modified Barthel Index, and EQ-5D-3L, were conducted before and after the
intervention and again at the 8-week mark following its completion. Repeated-measures ANOVA was used for comparisons between and within groups.
Results
Twenty-six patients were assessed for eligibility, and 20 were enrolled and randomized. No significant
differences were observed between the tDCS with gait training group and the sham stimulation group in
gait speed after the intervention. However, the tDCS with gait training group showed significant
improvement in balance performance in both within-group and between-group comparisons. In the
subgroup analysis of patients with elicited motor-evoked potentials, comfortable pace gait speed
improved in the tDCS with gait training group. No serious adverse events occurred throughout the study.
Conclusions
Simultaneous tDCS during gait training is a feasible rehabilitation protocol for chronic stroke patients
with gait disturbances.
Introduction
Impairment of independent gait is one of the most disabling consequences after a stroke [1]. Gait
abnormality in stroke patients arises from a complex interplay of factors, including lower limb motor
weakness and decreased balance. Some individuals may not be entirely incapable of walking, but they
may still require gait aids or assistance from caregivers. Gait disturbances pose a greater risk of further
injury due to falls. It is well known that fall-associated fractures result in significant socioeconomic costs
[2]. Additionally, gait disturbances lead to limitations in social activity, thereby reducing the quality of life
of stroke patients. Therefore, improving gait performance in stroke patients has long been a desire shared
by patients and physicians.
Transcranial direct current stimulation (tDCS) is a non-invasive brain stimulation technique that aims to
modulate the human brain by delivering low-intensity electrical current through the scalp. The mechanism of tDCS is explained by 2 principles: (1) the enhancement of cortical activity through a polarity shift in the resting membrane potential and (2) the upregulation of neural plasticity through long-term potentiation [3]. Applying anodal tDCS to patients with subacute stroke has been associated with beneficial effects on motor function [4]. However, inconsistent results have emerged across studies, with some failing to observe significant improvements in patients who underwent tDCS compared to sham stimulation [5].
Additionally, there has been substantial variability in factors, such as stimulation area, intensity, duration,
and the number of sessions among different tDCS protocols, highlighting the need for further research to
establish an optimal tDCS protocol for maximizing the effect of conventional gait training methods.
Studies focusing on stimulation timing suggest that combining tDCS with gait training simultaneously
shows more promising results in improving gait performance compared to protocols that administer gait
training and tDCS separately [6].
This study aimed to evaluate the feasibility of a rehabilitation protocol that combines simultaneous tDCS
with conventional gait training and to investigate its impact on gait performance in chronic stroke
patients.[…]
[LITERATURE REVIEW] Characteristics and Methodological Quality of the Top 50 Most Influential Articles on Stroke Rehabilitation
Posted by Kostas Pantremenos in REHABILITATION on March 24, 2024
A Bibliometric Analysis
Abstract
This study aimed to conduct a comprehensive review of the top 50 most influential articles on stroke rehabilitation to investigate characteristics, such as the number of citations, year of publication, study design, and research topic, as well as to assess the evidence level and methodological quality. Moreover, we performed a supplementary assessment of the top 10 articles published within the past 5 yrs in the same domain, aiming to discern potential shifts in trends and methodological quality. Web of Science was used to search for articles on stroke rehabilitation. The data extracted from the articles included title, journal impact factor, year of publication, total number of citations, article topic, study design, and others. The level of evidence and methodological quality were assessed by two reviewers. Noninvasive brain stimulation and robotic rehabilitation were frequently discussed in the top 50 articles. We found that there was no difference in methodology quality between the top 50 articles in all years and the top ten articles in the past 5 yrs. Furthermore, the number of citations and citation density were not associated with the methodological quality. The findings suggest that the number of citations alone may not be a reliable indicator of research quality.
Stroke patients experience motor paralysis as well as sensory and cognitive impairments that limit their activities of daily living and social activities.1 Recovery from stroke is most accelerated within the initial weeks but may exhibit further long-term improvement through rehabilitation.2,3 Various rehabilitation programs are offered to stroke patients targeting the enhancement of motor function, gait, and activities of their daily lives.4 Notably, in recent years, there has been a surge in studies focusing on interventions such as treadmill exercise,5 electrical stimulation,6 mental practice,7 robotic therapy,8 and virtual reality.9 The medical care landscape is evolving rapidly globally. In an era dominated by evidence-based medicine, maintaining familiarity with both historical and current advancements in rehabilitation and research is indispensable for informed clinical decision making.
Previous studies have explored the prevailing trends in high-impact articles across diverse diseases by leveraging the impact factor as an evaluation.10–13 Researchers often acknowledge the inherent ranking system of scholarly journals based on their impact factors and tailor their submission and reading habits accordingly.14 However, it is crucial to acknowledge that a substantial number of citations or publications in a high-impact journal does not necessarily guarantee the intrinsic quality of a study.15 For medical professionals to provide better quality evidence-based medicine to patients, it is necessary to understand the study design and the strength of the level of evidence and judge the quality of the study through proper critical appraisal.
Focusing specifically on stroke rehabilitation, Hu et al.16 conducted an extensive survey to elucidate the prevailing research trends pertaining to motor functions. However, their study falls short of discussing the methodological quality and level of evidence employed. Furthermore, their exclusive focus on motor function fails to encompass the entire rehabilitation landscape. Thus, the purpose of this study was to conduct a comprehensive review of the top 50 most influential articles on stroke rehabilitation to investigate characteristics, such as the number of citations, year of publication, study design, and research topic, as well as to assess the evidence level and methodological quality. The “most influential article” in this study was defined as the article with the most citations. Moreover, we performed a supplementary assessment of the top 10 articles published within the past 5 yrs in the same domain, aiming to discern potential shifts in trends and methodological quality. […]
[Review] Soft Hand Exoskeletons for Rehabilitation: Approaches to Design, Manufacturing Methods, and Future Prospects
Posted by Kostas Pantremenos in Paretic Hand, Rehabilitation robotics on March 24, 2024
Abstract
Stroke, the third leading cause of global disability, poses significant challenges to healthcare systems worldwide. Addressing the restoration of impaired hand functions is crucial, especially amid healthcare workforce shortages. While robotic-assisted therapy shows promise, cost and healthcare community concerns hinder the adoption of hand exoskeletons. However, recent advancements in soft robotics and digital fabrication, particularly 3D printing, have sparked renewed interest in this area. This review article offers a thorough exploration of the current landscape of soft hand exoskeletons, emphasizing recent advancements and alternative designs. It surveys previous reviews in the field and examines relevant aspects of hand anatomy pertinent to wearable rehabilitation devices. Furthermore, the article investigates the design requirements for soft hand exoskeletons and provides a detailed review of various soft exoskeleton gloves, categorized based on their design principles. The discussion encompasses simulation-supported methods, affordability considerations, and future research directions. This review aims to benefit researchers, clinicians, and stakeholders by disseminating the latest advances in soft hand exoskeleton technology, ultimately enhancing stroke rehabilitation outcomes and patient care.
1. Introduction
Strokes are the second leading cause of death worldwide [1,2,3]. These events, which consist of the sudden death of some brain cells due to lack of oxygen when blood flow is lost due to blockage or rupture of an artery [4,5], are also the third cause of permanent disability and one of the main causes of dementia and depression [6,7]. The occurrence of strokes is subject to different risk factors [8,9]. Diseases such as hypertension and diabetes, along with habits such as smoking, are considered modifiable risk factors, that is, factors susceptible to prevention. Other risk factors are not preventable, such as atrial fibrillation and transient ischemic attacks, which are presumed to be of genetic origin [10].
Globally, 70% of strokes and 87% of deaths related to these events occur in low- and middle-income countries [11]. Over the past four decades, the incidence of stroke in low- and middle-income countries has increased by more than 100%. During these decades, its incidence has decreased by 42% in high-income countries. On average, this event occurs 15 years earlier and causes more deaths for people living in low- and middle-income countries when compared to those in high-income countries. Strokes mainly affect people at the peak of their productive lives [12], and despite its enormous impact on the socio-economic development of countries, this growing crisis has received very little attention to date.
Recent neurological research indicates that impaired motor skills of stroke patients can be improved and possibly restored through repetitive, task-oriented training [13,14,15,16,17]. This is due to a property of the human brain known as neuroplasticity or the ability of the brain to reorganize itself by establishing new neural connections [18,19]. On this basis, the use of automatic devices has been implemented to help therapists increase the intensity of treatments, produce multisensory stimulation, and reduce costs during their work [20,21,22,23,24,25]. This new concept dates to the early 1990s with a new family of robotic machines called “haptic interfaces”. These mechanical devices were designed to interact with the human being, guiding the upper limb towards passive and active assisted mobilization, assisting in some movement tasks through biofeedback systems, and measuring kinematic changes and dynamics in motion. However, there is no consensus on the metrics or devices used to treat motor function deficiencies through neuroplasticity [26,27]. Additionally, recovery success relies heavily on a patient’s ability to attend therapy, which can be deterred by the frequency, duration, or cost of the therapy [28]. In this way, robotic therapy could represent a standard and successful complement to rehabilitation programs, improve patient access, and increase compliance and subsequent outcomes of rehabilitation efforts.
Despite the multiple designs reviewed in the last decade [29], there is no clinical evidence of the superiority of robotic training over traditional therapy. However, this procedure can complement traditional therapy processes [30]. In contrast, soft robots have the potential to overcome the limitations that are present in rigid robots. This design paradigm, inspired by the behavior and structure of living things, seems to be more compatible with rehabilitation activities due to increased versatility and compliance [31,32].
In addition, the solutions proposed, under the appropriate portability parameters, would have the potential to support the patient in the execution of activities of daily living, increasing their level of independence, mental health, and the well-being of their family nucleus.
The purpose of this work is to present a comparative review of the most recent rehabilitation hand exoskeletons based on soft actuators. This comparison is based on two main criteria: (i) the complexity of the manufacturing process and (ii) the resulting adaptability to the patient’s anatomy in rehabilitation environments.
This review also wants to motivate future designers to work in interdisciplinary solutions, taking into consideration multiple initiatives related to the implementation of makerspaces within the facilities of healthcare institutions, enabling the high reception and adoption of digital fabrication methods in society, and specifically, in healthcare [33,34,35,36,37,38]. […]

[Abstract] Survey of the clinical practices of physiotherapists for the management of post-stroke fatigue
Posted by Kostas Pantremenos in Fatigue on March 19, 2024
OBJECTIVE: Post-stroke fatigue (PSF) is a common condition among stroke survivors. However, evidence supporting the effective clinical management of PSF is insufficient. Our objectives were to examine the clinical practices of physiotherapists for the management of PSF and evaluate their clinical knowledge and confidence in managing PSF.
SUBJECTS AND METHODS: We conducted a cross-sectional study using an online survey of the sociodemographic profiles of participating physiotherapists, their current clinical practices, clinical knowledge, confidence in their clinical management of PSF, and the types and intensity of the exercises used in the management of PSF.
RESULTS: A total of 160 physiotherapists completed the survey: 86 (53.8%) were women, 148 (92.5%) were Saudi nationals, 126 (78.7%) were employed by the Ministry of Health, and 34 (21.3%) worked in the private sector. The majority (60%) of physiotherapists did not routinely assess their patients for the presence of fatigue. Likewise, 93 (58.1%) did not provide any PSF-related educational material to their patients; however, 67 (41.9%) did provide these materials. The preferred exercises of the physiotherapists for their patients were bed and chair exercises (59.5%), followed by functional training (51.4%), and resistance training (23.1%).
CONCLUSIONS: Our results suggest that while physiotherapists practicing in Saudi Arabia have a sound theoretical understanding of PSF management, their knowledge does not necessarily translate into practice. Interventions used to treat PSF include bed and chair exercises, functional training, and resistance training.
