Posts Tagged Motor
[Abstract + References] The effects of rTMS on motor recovery after stroke: a systematic review of fMRI studies
Posted by Kostas Pantremenos in tDCS/rTMS on November 12, 2023
Abstract
Repetitive transcranial magnetic stimulation (rTMS) has been widely used in motor rehabilitation after stroke, and functional magnetic resonance imaging (fMRI) has been used to investigate the neural mechanisms of motor recovery during stroke therapy. However, there is no review on the mechanism of rTMS intervention for motor recovery after stroke based on fMRI explicitly. We aim to reveal and summarize the neural mechanism of the effects of rTMS on motor function after stroke as measured by fMRI. We carefully performed a literature search using PubMed, EMBASE, Web of Science, and Cochrane Library databases from their respective inceptions to November 2022 to identify any relevant randomized controlled trials. Researchers independently screened the literature, extracted data, and qualitatively described the included studies. Eleven studies with a total of 420 poststroke patients were finally included in this systematic review. A total of 338 of those participants received fMRI examinations before and after rTMS intervention. Five studies reported the effects of rTMS on activation of brain regions, and four studies reported results related to brain functional connectivity (FC). Additionally, five studies analyzed the correlation between fMRI and motor evaluation. The neural mechanism of rTMS in improving motor function after stroke may be the activation and FCs of motor-related brain areas, including enhancement of the activation of motor-related brain areas in the affected hemisphere, inhibition of the activation of motor-related brain areas in the unaffected hemisphere, and changing the FCs of intra-hemispheric and inter-hemispheric motor networks.
This is a preview of subscription content, log in via an institution to check access.
Data availability
Data sharing is not applicable as no datasets were generated or analyzed in this study.
References
- Kuriakose D, Xiao Z (2020) Pathophysiology and treatment of stroke: present status and future perspectives. Int J Mol Sci 21(20). https://doi.org/10.3390/ijms21207609
- Peisker T, Koznar B, Stetkarova I, Widimsky P (2017) Acute stroke therapy: a review. Trends Cardiovasc Med 27(1):59–66. https://doi.org/10.1016/j.tcm.2016.06.009Article PubMed Google Scholar
- Gou X, Xu D, Li F, Hou K, Fang W, Li Y (2021) Pyroptosis in stroke-new insights into disease mechanisms and therapeutic strategies. J Physiol Biochem 77(4):511–529. https://doi.org/10.1007/s13105-021-00817-wArticle CAS PubMed Google Scholar
- Global, regional, and national burden of stroke, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016 (2019) Global, regional, and national burden of stroke, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 18(5):439–458. https://doi.org/10.1016/S1474-4422(19)30034-1Article Google Scholar
- Langhorne P, Coupar F, Pollock A (2009) Motor recovery after stroke: a systematic review. Lancet Neurol 8(8):741–754. https://doi.org/10.1016/S1474-4422(09)70150-4Article PubMed Google Scholar
- Stinear CM (2017) Prediction of motor recovery after stroke: advances in biomarkers. Lancet Neurol 16(10):826–836. https://doi.org/10.1016/S1474-4422(17)30283-1Article PubMed Google Scholar
- Somaa FA, de Graaf TA, Sack AT (2022) Transcranial magnetic stimulation in the treatment of neurological diseases. Front Neurol 13:793253. https://doi.org/10.3389/fneur.2022.793253Article PubMed PubMed Central Google Scholar
- Fisicaro F, Lanza G, Grasso AA, Pennisi G, Bella R, Paulus W, Pennisi M (2019) Repetitive transcranial magnetic stimulation in stroke rehabilitation: review of the current evidence and pitfalls. Ther Adv Neurol Disord 12:1278099885. https://doi.org/10.1177/1756286419878317Article Google Scholar
- Lefaucheur JP, Aleman A, Baeken C, Benninger DH, Brunelin J, Di Lazzaro V, Filipović SR, Grefkes C, Hasan A, Hummel FC, Jääskeläinen SK, Langguth B, Leocani L, Londero A, Nardone R, Nguyen JP, Nyffeler T, Oliveira-Maia AJ, Oliviero A, Padberg F, Palm U, Paulus W, Poulet E, Quartarone A, Rachid F, Rektorová I, Rossi S, Sahlsten H, Schecklmann M, Szekely D, Ziemann U (2020) Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS): an update (2014–2018). Clin Neurophysiol 131(2):474–528. https://doi.org/10.1016/j.clinph.2019.11.002Article PubMed Google Scholar
- Schambra HM (2018) Repetitive transcranial magnetic stimulation for upper extremity motor recovery: does it help? Curr Neurol Neurosci Rep 18(12):97. https://doi.org/10.1007/s11910-018-0913-8Article PubMed PubMed Central Google Scholar
- Kobayashi M, Pascual-Leone A (2003) Transcranial magnetic stimulation in neurology. Lancet Neurol 2(3):145–156. https://doi.org/10.1016/s1474-4422(03)00321-1Article PubMed Google Scholar
- Gilio F, Conte A, Vanacore N, Frasca V, Inghilleri M, Berardelli A (2007) Excitatory and inhibitory after-effects after repetitive magnetic transcranial stimulation (rTMS) in normal subjects. Exp Brain Res 176(4):588–593. https://doi.org/10.1007/s00221-006-0638-9Article CAS PubMed Google Scholar
- Tang Z, Han K, Wang R, Zhang Y, Zhang H (2022) Excitatory repetitive transcranial magnetic stimulation over the ipsilesional hemisphere for upper limb motor function after stroke: a systematic review and meta-analysis. Front Neurol 13:918597. https://doi.org/10.3389/fneur.2022.918597Article PubMed PubMed Central Google Scholar
- Bai Z, Zhang J, Fong K (2022) Effects of transcranial magnetic stimulation in modulating cortical excitability in patients with stroke: a systematic review and meta-analysis. J Neuroeng Rehabil 19(1):24. https://doi.org/10.1186/s12984-022-00999-4Article PubMed PubMed Central Google Scholar
- Gutiérrez-Muto AM, Castilla J, Freire M, Oliviero A, Tornero J (2020) Theta burst stimulation: technical aspects about TMS devices. Brain Stimul 13(3):562–564. https://doi.org/10.1016/j.brs.2020.01.002Article PubMed Google Scholar
- Suppa A, Huang YZ, Funke K, Ridding MC, Cheeran B, Di Lazzaro V, Ziemann U, Rothwell JC (2016) Ten years of theta burst stimulation in humans: established knowledge, unknowns and prospects. Brain Stimul 9(3):323–335. https://doi.org/10.1016/j.brs.2016.01.006Article CAS PubMed Google Scholar
- Huang YZ, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC (2005) Theta burst stimulation of the human motor cortex. Neuron 45(2):201–206. https://doi.org/10.1016/j.neuron.2004.12.033Article CAS PubMed Google Scholar
- Di Pino G, Pellegrino G, Assenza G, Capone F, Ferreri F, Formica D, Ranieri F, Tombini M, Ziemann U, Rothwell JC, Di Lazzaro V (2014) Modulation of brain plasticity in stroke: a novel model for neurorehabilitation. Nat Rev Neurol 10(10):597–608. https://doi.org/10.1038/nrneurol.2014.162Article PubMed Google Scholar
- Simonetta-Moreau M (2014) Non-invasive brain stimulation (NIBS) and motor recovery after stroke. Ann Phys Rehabil Med 57(8):530–542. https://doi.org/10.1016/j.rehab.2014.08.003Article CAS PubMed Google Scholar
- Stefan K, Kunesch E, Cohen LG, Benecke R, Classen J (2000) Induction of plasticity in the human motor cortex by paired associative stimulation. Brain 123(Pt 3):572–584. https://doi.org/10.1093/brain/123.3.572Article PubMed Google Scholar
- Guidali G, Roncoroni C, Bolognini N (2021) Modulating frontal networks’ timing-dependent-like plasticity with paired associative stimulation protocols: recent advances and future perspectives. Front Hum Neurosci 15:658723. https://doi.org/10.3389/fnhum.2021.658723Article PubMed PubMed Central Google Scholar
- Stefan K, Kunesch E, Benecke R, Cohen LG, Classen J (2002) Mechanisms of enhancement of human motor cortex excitability induced by interventional paired associative stimulation. J Physiol 543(Pt 2):699–708. https://doi.org/10.1113/jphysiol.2002.023317Article CAS PubMed PubMed Central Google Scholar
- Hu Y, Guo TC, Zhang XY, Tian J, Lu YS (2019) Paired associative stimulation improves synaptic plasticity and functional outcomes after cerebral ischemia. Neural Regen Res 14(11):1968–1976. https://doi.org/10.4103/1673-5374.259618Article CAS PubMed PubMed Central Google Scholar
- Carson RG, Kennedy NC (2013) Modulation of human corticospinal excitability by paired associative stimulation. Front Hum Neurosci 7:823. https://doi.org/10.3389/fnhum.2013.00823Article PubMed PubMed Central Google Scholar
- Du J, Tian L, Liu W, Hu J, Xu G, Ma M, Fan X, Ye R, Jiang Y, Yin Q, Zhu W, Xiong Y, Yang F, Liu X (2016) Effects of repetitive transcranial magnetic stimulation on motor recovery and motor cortex excitability in patients with stroke: a randomized controlled trial. Eur J Neurol 23(11):1666–1672. https://doi.org/10.1111/ene.13105Article CAS PubMed Google Scholar
- Moslemi HF, Kordi YA, Razeghi M, Shariat A, Bagheri Z, Rezaei K (2021) The effect of high-frequency repetitive transcranial magnetic stimulation on functional indices of affected upper limb in patients with subacute stroke. J Biomed Phys Eng 11(2):175–184. https://doi.org/10.31661/jbpe.v0i0.879Article Google Scholar
- Luk KY, Ouyang HX, Pang M (2022) Low-frequency rTMS over contralesional M1 increases ipsilesional cortical excitability and motor function with decreased interhemispheric asymmetry in subacute stroke: a randomized controlled study. Neural Plast 2022:3815357. https://doi.org/10.1155/2022/3815357Article PubMed PubMed Central Google Scholar
- Avenanti A, Coccia M, Ladavas E, Provinciali L, Ceravolo MG (2012) Low-frequency rTMS promotes use-dependent motor plasticity in chronic stroke: a randomized trial. Neurology 78(4):256–264. https://doi.org/10.1212/WNL.0b013e3182436558Article CAS PubMed Google Scholar
- Guo Z, Jin Y, Bai X, Jiang B, He L, McClure MA, Mu Q (2021) Distinction of high- and low-frequency repetitive transcranial magnetic stimulation on the functional reorganization of the motor network in stroke patients. Neural Plast 2021:8873221. https://doi.org/10.1155/2021/8873221Article PubMed PubMed Central Google Scholar
- Zhang JJ, Bai Z, Fong K (2022) Priming intermittent theta burst stimulation for hemiparetic upper limb after stroke: a randomized controlled trial. Stroke 53(7):2171–2181. https://doi.org/10.1161/STROKEAHA.121.037870Article PubMed Google Scholar
- Park CH, Chang WH, Ohn SH, Kim ST, Bang OY, Pascual-Leone A, Kim YH (2011) Longitudinal changes of resting-state functional connectivity during motor recovery after stroke. Stroke 42(5):1357–1362. https://doi.org/10.1161/STROKEAHA.110.596155Article PubMed PubMed Central Google Scholar
- Golestani AM, Tymchuk S, Demchuk A, Goodyear BG (2013) Longitudinal evaluation of resting-state FMRI after acute stroke with hemiparesis. Neurorehabil Neural Repair 27(2):153–163. https://doi.org/10.1177/1545968312457827Article PubMed Google Scholar
- Lam TK, Dawson DR, Honjo K, Ross B, Binns MA, Stuss DT, Black SE, Chen JJ, Levine BT, Fujioka T, Chen JL (2018) Neural coupling between contralesional motor and frontoparietal networks correlates with motor ability in individuals with chronic stroke. J Neurol Sci 384:21–29. https://doi.org/10.1016/j.jns.2017.11.007Article PubMed Google Scholar
- Peng Y, Liu J, Hua M, Liang M, Yu C (2019) Enhanced effective connectivity from ipsilesional to contralesional M1 in well-recovered subcortical stroke patients. Front Neurol 10:909. https://doi.org/10.3389/fneur.2019.00909Article PubMed PubMed Central Google Scholar
- Frahm J, Bruhn H, Merboldt KD, Hänicke W (1992) Dynamic MR imaging of human brain oxygenation during rest and photic stimulation. J Magn Reson Imaging 2(5):501–505. https://doi.org/10.1002/jmri.1880020505Article CAS PubMed Google Scholar
- Kwong KK, Belliveau JW, Chesler DA, Goldberg IE, Weisskoff RM, Poncelet BP, Kennedy DN, Hoppel BE, Cohen MS, Turner R, Et A (1992) Dynamic magnetic resonance imaging of human brain activity during primary sensory stimulation. Proc Natl Acad Sci USA 89(12):5675–5679. https://doi.org/10.1073/pnas.89.12.5675Article CAS PubMed PubMed Central Google Scholar
- Turner R, Howseman A, Rees GE, Josephs O, Friston K (1998) Functional magnetic resonance imaging of the human brain: data acquisition and analysis. Exp Brain Res 123(1–2):5–12. https://doi.org/10.1007/s002210050538Article CAS PubMed Google Scholar
- Li B, Deng S, Sang B, Zhu W, Zhuo B, Zhang M, Qin C, Lyu Y, Du Y, Meng Z (2022) Revealing the neuroimaging mechanism of acupuncture for poststroke aphasia: a systematic review. Neural Plast 2022:5635596. https://doi.org/10.1155/2022/5635596Article PubMed PubMed Central Google Scholar
- Fox MD, Raichle ME (2007) Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci 8(9):700–711. https://doi.org/10.1038/nrn2201Article CAS PubMed Google Scholar
- Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Plos Med 6(7):e1000097. https://doi.org/10.1371/journal.pmed.1000097Article PubMed PubMed Central Google Scholar
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372:n71. https://doi.org/10.1136/bmj.n71Article PubMed PubMed Central Google Scholar
- Maher CG, Sherrington C, Herbert RD, Moseley AM, Elkins M (2003) Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys Ther 83(8):713–721Article PubMed Google Scholar
- de Morton NA (2009) The PEDro scale is a valid measure of the methodological quality of clinical trials: a demographic study. Aust J Physiother 55(2):129–133. https://doi.org/10.1016/s0004-9514(09)70043-1Article PubMed Google Scholar
- Ameli M, Grefkes C, Kemper F, Riegg FP, Rehme AK, Karbe H, Fink GR, Nowak DA (2009) Differential effects of high-frequency repetitive transcranial magnetic stimulation over ipsilesional primary motor cortex in cortical and subcortical middle cerebral artery stroke. Ann Neurol 66(3):298–309. https://doi.org/10.1002/ana.21725Article PubMed Google Scholar
- Chang WH, Kim YH, Yoo WK, Goo KH, Park CH, Kim ST, Pascual-Leone A (2012) rTMS with motor training modulates cortico-basal ganglia-thalamocortical circuits in stroke patients. Restor Neurol Neurosci 30(3):179–189. https://doi.org/10.3233/RNN-2012-110162Article PubMed PubMed Central Google Scholar
- Chen Q, Shen W, Sun H, Zhang H, Liu C, Chen Z, Yu L, Cai X, Ke J, Li L, Zhang L, Fang Q (2022) The effect of coupled inhibitory-facilitatory repetitive transcranial magnetic stimulation on shaping early reorganization of the motor network after stroke. Brain Res 1790:147959. https://doi.org/10.1016/j.brainres.2022.147959Article CAS PubMed Google Scholar
- Du J, Yang F, Hu J, Hu J, Xu Q, Cong N, Zhang Q, Liu L, Mantini D, Zhang Z, Lu G, Liu X (2019) Effects of high- and low-frequency repetitive transcranial magnetic stimulation on motor recovery in early stroke patients: evidence from a randomized controlled trial with clinical, neurophysiological and functional imaging assessments. Neuroimage Clin 21:101620. https://doi.org/10.1016/j.nicl.2018.101620Article PubMed Google Scholar
- Juan D, Yao W, Li J, Yang F, Hu J, Xu Q, Liu L, Lv Q, Liu R, Ye R, Ma M, Zhu W, Zhang Z, Liu X (2022) Motor network reorganization after repetitive transcranial magnetic stimulation in early stroke patients: a resting state fMRI study. Neurorehabil Neural Repair 36(1):61–68. https://doi.org/10.1177/15459683211054184Article Google Scholar
- Gottlieb A, Boltzmann M, Schmidt SB, Gutenbrunner C, Krauss JK, Stangel M, Höglinger GU, Wallesch CW, Rollnik JD (2021) Treatment of upper limb spasticity with inhibitory repetitive transcranial magnetic stimulation: a randomized placebo-controlled trial. NeuroRehabilitation 49(3):425–434. https://doi.org/10.3233/NRE-210088Article PubMed Google Scholar
- Li J, Zhang XW, Zuo ZT, Lu J, Meng CL, Fang HY, Xue R, Fan Y, Guan YZ, Zhang WH (2016) Cerebral functional reorganization in ischemic stroke after repetitive transcranial magnetic stimulation: an fMRI study. Cns Neurosci Ther 22(12):952–960. https://doi.org/10.1111/cns.12593Article CAS PubMed PubMed Central Google Scholar
- Nowak DA, Grefkes C, Dafotakis M, Eickhoff S, Küst J, Karbe H, Fink GR (2008) Effects of low-frequency repetitive transcranial magnetic stimulation of the contralesional primary motor cortex on movement kinematics and neural activity in subcortical stroke. Arch Neurol 65(6):741–747. https://doi.org/10.1001/archneur.65.6.741Article PubMed Google Scholar
- Qin Y, Liu X, Guo X, Liu M, Li H, Xu S (2021) Low-frequency repetitive transcranial magnetic stimulation restores dynamic functional connectivity in subcortical stroke. Front Neurol 12:771034. https://doi.org/10.3389/fneur.2021.771034Article PubMed PubMed Central Google Scholar
- Allen EA, Damaraju E, Plis SM, Erhardt EB, Eichele T, Calhoun VD (2014) Tracking whole-brain connectivity dynamics in the resting state. Cereb Cortex 24:663–676. https://doi.org/10.1093/cercor/bhs352Article PubMed Google Scholar
- Dekhil O, Shalaby A, Soliman A, Mahmoud A, Kong M, Barnes G, Elmaghraby A, El-Baz A (2021) Identifying brain areas correlated with ADOS raw scores by studying altered dynamic functional connectivity patterns. Med Image Anal 68:101899. https://doi.org/10.1016/j.media.2020.101899Article PubMed Google Scholar
- Rosso C, Moulton EJ, Kemlin C, Leder S, Corvol JC, Mehdi S, Obadia MA, Obadia M, Yger M, Meseguer E, Perlbarg V, Valabregue R, Magno S, Lindberg P, Meunier S, Lamy JC (2022) Cerebello-motor paired associative stimulation and motor recovery in stroke: a randomized, sham-controlled, double-blind pilot trial. Neurotherapeutics 19(2):491–500. https://doi.org/10.1007/s13311-022-01205-yArticle PubMed PubMed Central Google Scholar
- Tosun A, Türe S, Askin A, Yardimci EU, Demirdal SU, Kurt IT, Tosun O, Kocyigit H, Akhan G, Gelal FM (2017) Effects of low-frequency repetitive transcranial magnetic stimulation and neuromuscular electrical stimulation on upper extremity motor recovery in the early period after stroke: a preliminary study. Top Stroke Rehabil 24(5):361–367. https://doi.org/10.1080/10749357.2017.1305644Article PubMed Google Scholar
- Zhang L, Xing G, Fan Y, Guo Z, Chen H, Mu Q (2017) Short- and long-term effects of repetitive transcranial magnetic stimulation on upper limb motor function after stroke: a systematic review and meta-analysis. Clin Rehabil 31(9):1137–1153. https://doi.org/10.1177/0269215517692386Article PubMed Google Scholar
- Xiang H, Sun J, Tang X, Zeng K, Wu X (2019) The effect and optimal parameters of repetitive transcranial magnetic stimulation on motor recovery in stroke patients: a systematic review and meta-analysis of randomized controlled trials. Clin Rehabil 33(5):847–864. https://doi.org/10.1177/0269215519829897Article PubMed Google Scholar
- Fan H, Song Y, Cen X, Yu P, Bíró I, Gu Y (2021) The effect of repetitive transcranial magnetic stimulation on lower-limb motor ability in stroke patients: a systematic review. Front Hum Neurosci 15:620573. https://doi.org/10.3389/fnhum.2021.620573Article PubMed PubMed Central Google Scholar
- Gao B, Wang Y, Zhang D, Wang Z, Wang Z (2022) Intermittent theta-burst stimulation with physical exercise improves poststroke motor function: a systemic review and meta-analysis. Front Neurol 13:964627. https://doi.org/10.3389/fneur.2022.964627Article PubMed PubMed Central Google Scholar
- Liepert J, Hamzei F, Weiller C (2000) Motor cortex disinhibition of the unaffected hemisphere after acute stroke. Muscle Nerve 23(11):1761–1763. https://doi.org/10.1002/1097-4598(200011)23:11%3c1761::aid-mus14%3e3.0.co;2-mArticle CAS PubMed Google Scholar
- Shimizu T, Hosaki A, Hino T, Sato M, Komori T, Hirai S, Rossini PM (2002) Motor cortical disinhibition in the unaffected hemisphere after unilateral cortical stroke. Brain 125(Pt 8):1896–1907. https://doi.org/10.1093/brain/awf183Article PubMed Google Scholar
- Cicinelli P, Pasqualetti P, Zaccagnini M, Traversa R, Oliveri M, Rossini PM (2003) Interhemispheric asymmetries of motor cortex excitability in the postacute stroke stage: a paired-pulse transcranial magnetic stimulation study. Stroke 34(11):2653–2658. https://doi.org/10.1161/01.STR.0000092122.96722.72Article PubMed Google Scholar
- Murase N, Duque J, Mazzocchio R, Cohen LG (2004) Influence of interhemispheric interactions on motor function in chronic stroke. Ann Neurol 55(3):400–409. https://doi.org/10.1002/ana.10848Article PubMed Google Scholar
- Koski L, Mernar TJ, Dobkin BH (2004) Immediate and long-term changes in corticomotor output in response to rehabilitation: correlation with functional improvements in chronic stroke. Neurorehabil Neural Repair 18(4):230–249. https://doi.org/10.1177/1545968304269210Article PubMed Google Scholar
- Lin YL, Potter-Baker KA, Cunningham DA, Li M, Sankarasubramanian V, Lee J, Jones S, Sakaie K, Wang X, Machado AG, Plow EB (2020) Stratifying chronic stroke patients based on the influence of contralesional motor cortices: an inter-hemispheric inhibition study. Clin Neurophysiol 131(10):2516–2525. https://doi.org/10.1016/j.clinph.2020.06.016Article PubMed PubMed Central Google Scholar
- Bocci T, Pietrasanta M, Cerri C, Restani L, Caleo M, Sartucci F (2014) Visual callosal connections: role in visual processing in health and disease. Rev Neurosci 25(1):113–127. https://doi.org/10.1515/revneuro-2013-0025Article PubMed Google Scholar
- Bocci T, Nasini F, Caleo M, Restani L, Barloscio D, Ardolino G, Priori A, Maffei L, Nardi M, Sartucci F (2018) Unilateral application of cathodal tDCS reduces transcallosal inhibition and improves visual acuity in amblyopic patients. Front Behav Neurosci 12:109. https://doi.org/10.3389/fnbeh.2018.00109Article PubMed PubMed Central Google Scholar
- Wang Q, Zhang D, Zhao Y, Hai H, Ma Y (2020) Effects of high-frequency repetitive transcranial magnetic stimulation over the contralesional motor cortex on motor recovery in severe hemiplegic stroke: a randomized clinical trial. Brain Stimul 13:979–986. https://doi.org/10.1016/j.brs.2020.03.020Article PubMed Google Scholar
- Grefkes C, Fink GR (2012) Disruption of motor network connectivity post-stroke and its noninvasive neuromodulation. Curr Opin Neurol 25(6):670–675. https://doi.org/10.1097/WCO.0b013e3283598473Article PubMed Google Scholar
- Paul T, Hensel L, Rehme AK, Tscherpel C, Eickhoff SB, Fink GR, Grefkes C, Volz LJ (2021) Early motor network connectivity after stroke: an interplay of general reorganization and state-specific compensation. Hum Brain Mapp 42(16):5230–5243. https://doi.org/10.1002/hbm.25612Article PubMed PubMed Central Google Scholar
- Olafson ER, Jamison KW, Sweeney EM, Liu H, Wang D, Bruss JE, Boes AD, Kuceyeski A (2021) Functional connectome reorganization relates to post-stroke motor recovery and structural and functional disconnection. Neuroimage 245:118642. https://doi.org/10.1016/j.neuroimage.2021.118642Article PubMed Google Scholar
- Hartwigsen G, Volz LJ (2021) Probing rapid network reorganization of motor and language functions via neuromodulation and neuroimaging. Neuroimage 224:117449. https://doi.org/10.1016/j.neuroimage.2020.117449Article PubMed Google Scholar
- Binder E, Leimbach M, Pool EM, Volz LJ, Eickhoff SB, Fink GR, Grefkes C (2021) Cortical reorganization after motor stroke: a pilot study on differences between the upper and lower limbs. Hum Brain Mapp 42(4):1013–1033. https://doi.org/10.1002/hbm.25275Article PubMed Google Scholar
- Rogers BP, Morgan VL, Newton AT, Gore JC (2007) Assessing functional connectivity in the human brain by fMRI. Magn Reson Imaging 25(10):1347–1357. https://doi.org/10.1016/j.mri.2007.03.007Article PubMed PubMed Central Google Scholar
- van den Heuvel MP, Hulshoff PH (2010) Exploring the brain network: a review on resting-state fMRI functional connectivity. Eur Neuropsychopharmacol 20(8):519–534. https://doi.org/10.1016/j.euroneuro.2010.03.008Article CAS PubMed Google Scholar
- Desowska A, Turner DL (2019) Dynamics of brain connectivity after stroke. Rev Neurosci 30(6):605–623. https://doi.org/10.1515/revneuro-2018-0082Article PubMed Google Scholar
- Xia Y, Huang G, Quan X, Qin Q, Li H, Xu C, Liang Z (2021) Dynamic structural and functional reorganizations following motor stroke. Med Sci Monit 27:e929092. https://doi.org/10.12659/MSM.929092Article PubMed PubMed Central Google Scholar
- Volz LJ, Rehme AK, Michely J, Nettekoven C, Eickhoff SB, Fink GR, Grefkes C (2016) Shaping early reorganization of neural networks promotes motor function after stroke. Cereb Cortex 26(6):2882–2894. https://doi.org/10.1093/cercor/bhw034Article CAS PubMed PubMed Central Google Scholar
- Li S, Francisco GE, Rymer WZ (2021) A new definition of poststroke spasticity and the interference of spasticity with motor recovery from acute to chronic stages. Neurorehabil Neural Repair 35(7):601–610. https://doi.org/10.1177/15459683211011214Article PubMed Google Scholar
[Abstract + References] Effects of Brain-Computer Interface Controlled Functional Electrical Stimulation on Motor Recovery in Stroke Survivors: a Systematic Review
Posted by Kostas Pantremenos in Functional Electrical Stimulation (FES) on September 20, 2022
Abstract
Purpose of Review
This systematic review aimed to investigate the effects of BCI-FES on motor recovery in patients with stroke.
Recent Findings
Nine studies met the eligibility criteria. Six studies were randomized controlled trials, and three were pilot studies. To date, the effectiveness of BCI-FES in patients with stroke has not been systematically reviewed.
Summary
The BCI-FES intervention may improve upper extremity function post-stroke. There is moderate evidence for positive effects of BCI-FES on gait and weak evidence for positive effects of BCI-FES on balance post-stroke. Further randomized controlled trials with a larger sample size are strongly warranted to confirm our findings.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- Thrift AG, Howard G, Cadilhac DA, et al. Global stroke statistics: an update of mortality data from countries using a broad code of “cerebrovascular diseases.” Int J Stroke. 2017;12(8):796–801. https://doi.org/10.1177/1747493017730782.Article PubMed Google Scholar
- Langhorne P, Coupar F, Pollock A. Motor recovery after stroke: a systematic review. Lancet Neurol. 2009;8(8):741–54. https://doi.org/10.1016/S1474-4422(09)70150-4.Article PubMed Google Scholar
- Kiper P, Szczudlik A, Agostini M, et al. Virtual reality for upper limb rehabilitation in subacute and chronic stroke: a randomized controlled trial. Arch Phys Med Rehabil. 2018;99(5):834-842.e4. https://doi.org/10.1016/j.apmr.2018.01.023.Article PubMed Google Scholar
- Dorsch S, Ada L, Canning CG, Al-Zharani M, Dean C. The strength of the ankle dorsiflexors has a significant contribution to walking speed in people who can walk independently after stroke: an observational study. Arch Phys Med Rehabil. 2012;93(6):1072–6. https://doi.org/10.1016/j.apmr.2012.01.005.Article PubMed Google Scholar
- Robertson JA, Eng JJ, Hung C. The effect of functional electrical stimulation on balance function and balance confidence in community-dwelling individuals with stroke. Physiother Can. 2010;62(2):114–9. https://doi.org/10.3138/physio.62.2.114.Article PubMed PubMed Central Google Scholar
- Daly JJ. Response of gait deficits to neuromuscular electrical stimulation for stroke survivors. Expert Rev Neurother. 2006;6(10):1511–22. https://doi.org/10.1586/14737175.6.10.1511.Article PubMed Google Scholar
- Hendricks HT, van Limbeek J, Geurts AC, Zwarts MJ. Motor recovery after stroke: a systematic review of the literature. Arch Phys Med Rehabil. 2002;83(11):1629–37. https://doi.org/10.1053/apmr.2002.35473.Article PubMed Google Scholar
- Kim T, Kim S, Lee B. Effects of action observational training plus brain-computer interface-based functional electrical stimulation on paretic arm motor recovery in patient with stroke: a randomized controlled trial. Occup Ther Int. 2016;23(1):39–47. https://doi.org/10.1002/oti.1403.Article PubMed Google Scholar
- • Rathee D, Chowdhury A, Meena YK, Dutta A, McDonough S, Prasad G. Brain-machine interface-driven post-stroke upper-limb functional recovery correlates with beta-band mediated cortical networks. IEEE Trans Neural Syst Rehabil Eng. 2019;27(5):1020–31. https://doi.org/10.1109/TNSRE.2019.2908125. This study demonstrated that BCI is used to induce plasticity according to activity by paying attention to tasks requiring individuals to activate or deactivate specific brain regions.Article PubMed Google Scholar
- Collinger JL, Vinjamuri R, Degenhart AD, et al. Motor-related brain activity during action observation: a neural substrate for electrocorticographic brain-computer interfaces after spinal cord injury. Front Integr Neurosci. 2014;8:17. Published 2014 Feb 19. https://doi.org/10.3389/fnint.2014.00017
- McFarland DJ, Wolpaw JR. Brain-computer interfaces for communication and control. Commun ACM. 2011;54(5):60–6. https://doi.org/10.1145/1941487.1941506.Article PubMed PubMed Central Google Scholar
- Etoh S, Noma T, Takiyoshi Y, et al. Effects of repetitive facilitative exercise with neuromuscular electrical stimulation, vibratory stimulation and repetitive transcranial magnetic stimulation of the hemiplegic hand in chronic stroke patients. Int J Neurosci. 2016;126(11):1007–12. https://doi.org/10.3109/00207454.2015.1094473.Article PubMed Google Scholar
- Peckham PH, Knutson JS. Functional electrical stimulation for neuromuscular applications. Annu Rev Biomed Eng. 2005;7:327–60. https://doi.org/10.1146/annurev.bioeng.6.040803.140103.CAS Article PubMed Google Scholar
- Dobkin BH, Dorsch A. New evidence for therapies in stroke rehabilitation. Curr Atheroscler Rep. 2013;15(6):331. https://doi.org/10.1007/s11883-013-0331-y.Article PubMed PubMed Central Google Scholar
- Urrútia G, Bonfill X. Declaración PRISMA: una propuesta para mejorar la publicación de revisiones sistemáticas y metaanálisis. Med Clin. 2010;135(11):507–11. https://doi.org/10.1016/j.medcli.2010.01.015.Article Google Scholar
- Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100. https://doi.org/10.1371/journal.pmed.1000100.Article PubMed PubMed Central Google Scholar
- Maher CG, Sherrington C, Herbert RD, Moseley AM, Elkins M. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys Ther. 2003;83(8):713–21.Article Google Scholar
- Moher D, Cook DJ, Jadad AR, et al. Assessing the quality of reports of randomised trials: implications for the conduct of meta-analyses. Health Technol Assess. 1999;3(12):i–98.CAS Article Google Scholar
- PEDro score – Strokengine. Strokengine.ca. https://strokengine.ca/en/glossary/pedro-score/. Published 2021. Accessed August 24, 2021.
- Lee SH, Kim SS, Lee BH. Action observation training and brain-computer interface controlled functional electrical stimulation enhance upper extremity performance and cortical activation in patients with stroke: a randomized controlled trial. Physiother Theory Pract. 2020;1–9. https://doi.org/10.1080/09593985.2020.1831114
- Chung E, Lee BH, Hwang S. Therapeutic effects of brain-computer interface-controlled functional electrical stimulation training on balance and gait performance for stroke: a pilot randomized controlled trial. Med (Baltimore). 2020;99(51):e22612. https://doi.org/10.1097/MD.0000000000022612.CAS Article Google Scholar
- Chung E, Park SI, Jang YY, Lee BH. Effects of brain-computer interface-based functional electrical stimulation on balance and gait function in patients with stroke: preliminary results. J Phys Ther Sci. 2015;27(2):513–6. https://doi.org/10.1589/jpts.27.513.Article PubMed PubMed Central Google Scholar
- Biasiucci A, Leeb R, Iturrate I, et al. Brain-actuated functional electrical stimulation elicits lasting arm motor recovery after stroke. Nat Commun. 2018;9(1):2421. https://doi.org/10.1038/s41467-018-04673-z.CAS Article PubMed PubMed Central Google Scholar
- Remsik AB, Dodd K, Williams L Jr, et al. Behavioral outcomes following brain-computer interface intervention for upper extremity rehabilitation in stroke: a randomized controlled trial. Front Neurosci. 2018;12:752. https://doi.org/10.3389/fnins.2018.00752.Article PubMed PubMed Central Google Scholar
- Jang YY, Kim TH, Lee BH. Effects of Brain-computer interface-controlled functional electrical stimulation training on shoulder subluxation for patients with stroke: a randomized controlled trial. Occup Ther Int. 2016;23(2):175–85. https://doi.org/10.1002/oti.1422.Article PubMed Google Scholar
- Young BM, Nigogosyan Z, Walton LM, et al. 2015 Dose-response relationships using brain-computer interface technology impact stroke rehabilitation. Front Hum Neurosci. 2015;9:361. https://doi.org/10.3389/fnhum.2015.00361.Article PubMed PubMed Central Google Scholar
- McCrimmon CM, King CE, Wang PT, Cramer SC, Nenadic Z, Do AH. Brain-controlled functional electrical stimulation therapy for gait rehabilitation after stroke: a safety study. J Neuroeng Rehabil. 2015;12:57. https://doi.org/10.1186/s12984-015-0050-4.Article PubMed PubMed Central Google Scholar
- •• Kruse A, Suica Z, Taeymans J, Schuster-Amft C. Effect of brain-computer interface training based on non-invasive electroencephalography using motor imagery on functional recovery after stroke – a systematic review and meta-analysis. BMC Neurol. 2020;20(1):385. https://doi.org/10.1186/s12883-020-01960-5. This review demonstrated an improvement in functional recovery post-stroke after BCI combined with conventional therapy for a duration of four weeks or longer, with a preference for high-intensity training of five times per week.Article PubMed PubMed Central Google Scholar
- Grefkes C, Fink GR. Connectivity-based approaches in stroke and recovery of function. Lancet Neurol. 2014;13(2):206–16. https://doi.org/10.1016/S1474-4422(13)70264-3.Article PubMed Google Scholar
- Wu J, Quinlan EB, Dodakian L, et al. Connectivity measures are robust biomarkers of cortical function and plasticity after stroke. Brain. 2015;138(Pt 8):2359–69. https://doi.org/10.1093/brain/awv156.Article PubMed PubMed Central Google Scholar
- Nicolo P, Rizk S, Magnin C, Pietro MD, Schnider A, Guggisberg AG. Coherent neural oscillations predict future motor and language improvement after stroke. Brain. 2015;138(Pt 10):3048–60. https://doi.org/10.1093/brain/awv200.Article PubMed Google Scholar
- Pundik S, McCabe JP, Hrovat K, et al. Recovery of post stroke proximal arm function, driven by complex neuroplastic bilateral brain activation patterns and predicted by baseline motor dysfunction severity. Front Hum Neurosci. 2015;9:394. https://doi.org/10.3389/fnhum.2015.00394.Article PubMed PubMed Central Google Scholar
- Schaechter JD, Moore CI, Connell BD, Rosen BR, Dijkhuizen RM. Structural and functional plasticity in the somatosensory cortex of chronic stroke patients. Brain. 2006;129(Pt 10):2722–33. https://doi.org/10.1093/brain/awl214.Article PubMed Google Scholar
- • Bouton CE. Merging brain-computer interface and functional electrical stimulation technologies for movement restoration. Handb Clin Neurol. 2020;168:303–9. https://doi.org/10.1016/B978-0-444-63934-9.00022-6. This review showed that BCI-FES treatment involves repeated attempts at functional activities to actively modulate brain activity during imagined movement, resulting in reward-based and use-dependent reinforcements and induction of neuroplastic change in the disrupted motor system.Article PubMed Google Scholar
- Alashram AR, Annino G, Padua E. Robot-assisted gait training in individuals with spinal cord injury: A systematic review for the clinical effectiveness of Lokomat. J Clin Neurosci. 2021;91:260–9. https://doi.org/10.1016/j.jocn.2021.07.019.CAS Article PubMed Google Scholar
- Alashram AR, Padua E, Hammash AK, Lombardo M, Annino G. Effectiveness of virtual reality on balance ability in individuals with incomplete spinal cord injury: A systematic review. J Clin Neurosci. 2020;72:322–7. https://doi.org/10.1016/j.jocn.2020.01.037.Article PubMed Google Scholar
- Alashram AR, Padua E, Annino G. Effects of whole-body vibration on motor impairments in patients with neurological disorders: a systematic review. Am J Phys Med Rehabil. 2019;98(12):1084–98. https://doi.org/10.1097/PHM.0000000000001252.Article PubMed Google Scholar
- Annino G, Alashram AR, Alghwiri AA, et al. Effect of segmental muscle vibration on upper extremity functional ability poststroke: A randomized controlled trial. Med (Baltimore). 2019;98(7):e14444. https://doi.org/10.1097/MD.0000000000014444.Article Google Scholar
- Alashram AR, Padua E, Romagnoli C, Annino G. Effectiveness of focal muscle vibration on hemiplegic upper extremity spasticity in individuals with stroke: A systematic review. NeuroRehabilitation. 2019;45(4):471–81. https://doi.org/10.3233/NRE-192863.Article PubMed Google Scholar
- Alashram A, Annino G, Al-qtaishat M, Padua E. Mental practice combined with physical practice to enhance upper extremity functional ability poststroke: a systematic review. J Stroke Med. 2020;3(2):51–61. https://doi.org/10.1177/2516608520943793.Article Google Scholar
- Alashram A. Optimizing gait ability after task-oriented circuit class training in posttraumatic brain injury: a case report. Indian J Phys Med Rehabil. 2019;30(4):112–6. https://doi.org/10.5005/jp-journals-10066-0053.Article Google Scholar
- Alashram A, Annino G, Mercuri N. Task-oriented motor learning in upper extremity rehabilitation post stroke. J Stroke Med. 2019;2(2):95–104. https://doi.org/10.1177/2516608519864760.Article Google Scholar
- Alashram AR, Annino G, Mercuri NB. Changes in spasticity following functional electrical stimulation cycling in patients with spinal cord injury: a systematic review [published online ahead of print, 2020 May 14]. J Spinal Cord Med. 2020;1–14. https://doi.org/10.1080/10790268.2020.1763713
- Alashram A, Alghwiri A, Padua E, Annino G. Efficacy of proprioceptive neuromuscular facilitation on spasticity in patients with stroke: a systematic review. Phys Ther Rev. 2021;26(3):168–76. https://doi.org/10.1080/10833196.2021.1892281.Article Google Scholar
- Alashram AR, Annino G, Mercuri NB. Rhythmic auditory stimulation in gait rehabilitation for traumatic brain and spinal cord injury. J Clin Neurosci. 2019;69:287–8. https://doi.org/10.1016/j.jocn.2019.08.080.Article PubMed Google Scholar
- Egger M, Smith G. meta-analysis bias in location and selection of studies. BMJ. 1998;316(7124):61–6. https://doi.org/10.1136/bmj.316.7124.61.CAS Article PubMed PubMed Central Google Scholar
- Higgins J, Green S. Cochrane handbook of systematic reviews of interventions. Chichester: Wiley; 2008. p. 187–241.
[Abstract] Design of Elbow Exoskeleton with Wireless System Control for Post Stroke Flexion-Extension Rehabilitation – Conference Publication
Posted by Kostas Pantremenos in Paretic Hand, REHABILITATION, Rehabilitation robotics on January 25, 2022
Abstract
In this paper we proposed a rehabilitation system that assists therapists to restore motor function by repetitive flexion-extension movement using robotic technology. The sys-tem is an elbow exoskeleton that can be controlled based on elbow rotation angle and angular velocity. The system also equipped with wireless communication to give therapists an ability to determine the trajectory of motor movement patterns and speed. For speed control, therapists need to input Pulse Width Modulation (PWM) value by Android application that conducts 100% accuracy to communicate the PWM value to microcontroller through bluetooth connection. INA226 sensor used by the system for measuring current value in order to detect the voluntary movement that occured in subject’s arm. Proportional Integral Derivative control (PID) used in order to achieve a predetermined trajectory value and maintain the Rotation per Minute (RPM) of the DC motor. As a result, the system can reach the target for fulfill 120-degree movement with 83.3% accuracy with passive hand and 100% accuracy with active arm condition. The measure of the sensor is used to analyze the rehabilitation process quantitatively and determine future treatment. This method expected can help patients to get intensive rehabilitation and make it easier for therapists to evaluate and rehabilitate patients.
[ARTICLE] Exploring the Use of Brain-Computer Interfaces in Stroke Neurorehabilitation – Full Text
Posted by Kostas Pantremenos in Artificial intelligence, REHABILITATION on July 17, 2021
Abstract
With the continuous development of artificial intelligence technology, “brain-computer interfaces” are gradually entering the field of medical rehabilitation. As a result, brain-computer interfaces (BCIs) have been included in many countries’ strategic plans for innovating this field, and subsequently, major funding and talent have been invested in this technology. In neurological rehabilitation for stroke patients, the use of BCIs opens up a new chapter in “top-down” rehabilitation. In our study, we first reviewed the latest BCI technologies, then presented recent research advances and landmark findings in BCI-based neurorehabilitation for stroke patients. Neurorehabilitation was focused on the areas of motor, sensory, speech, cognitive, and environmental interactions. Finally, we summarized the shortcomings of BCI use in the field of stroke neurorehabilitation and the prospects for BCI technology development for rehabilitation.
1. Introduction
According to WHO clinical criteria, stroke is defined as “a rapidly developing sign of focal (or global) brain dysfunction lasting more than 24 hours (unless interrupted by death), with no apparent nonvascular cause.” Stroke is the world’s second leading cause of death and third leading cause of injury and can cause severe cognitive, emotional, and sensorimotor impairment in patients [1]. Most stroke victims survive the initial event, and the greatest impact of stroke disease is usually the long-term effect it has on the patient and their family [2, 3]. Unfortunately, there are significant gaps between countries in the quality of stroke research and the effectiveness of medical interventions [4]. Over the last decade, advances in the medical treatment of stroke patients have resulted in a substantial reduction in mortality rates. However, one-third of the 16 million patients worldwide remain disabled each year [5].
In traditional rehabilitation, the gold standard in care for poststroke recovery is a combination of specialized training and general aerobic exercise. Bimanual arm training (BAT) and constraint-induced movement therapy (CIMT) are two of the most established methods for treating stroke-related sports injuries [6]. These rehabilitation techniques are bottom-up interventions that focus on distal limb modulation to cause subsequent improvements in the neural circuits involved in motor recovery. However, even with intensive task-specific training and physical activity, 15-30% of people who have had a stroke are permanently disabled. As a result, many bottom-up interventions are ineffective in stroke patients who have very limited upper limb mobility (Fugl-Meyer score 20) [7]. We need to explore and develop more effective stroke rehabilitation strategies that supplement or replace traditional rehabilitation training.
The remodeling of neurological function after stroke may facilitate the development of new interventions for poststroke rehabilitation, and recent therapeutic options have shifted to facilitating neural circuit reorganization in order to restore motor function. These top-down approaches to rehabilitation are largely due to the mechanisms of brain plasticity [8]. The advancement of artificial intelligence methods and a better understanding of brain plasticity are also critical for functional movement recovery. The human brain’s ability to adapt to change and environmental stimuli (brain injury, treatment, and experience) by reorganizing its structure, function, and connections is known as brain plasticity [9]. The basic structural reserve and anatomical plasticity of the brain are important parameters for significant motor recovery [10]. Therefore, the key challenge is to figure out how to optimize neuroplasticity during treatment while also reinforcing connections across the infarcted region and promote creation of new connections, thus facilitating long-term functional recovery.
With the advancement of science and technology, artificial intelligence technologies, such as brain-computer interface (BCI), virtual reality (VR), and augmented reality (AR), are rapidly developing and are gradually being applied in the field of medicine. Due to its direct action on the brain, BCI induces brain plasticity and promotes functional reorganization of the brain, proving to be a superior approach in poststroke rehabilitation, especially for improving motor function in stroke patients. The limited neurorehabilitation modalities are no longer adequate to meet increasing rehabilitation needs of patients with central injuries, and BCI has been shown to be effective in improving motor function and enhancing the lives of stroke patients. In this review, we first examined the latest BCI technologies, including how BCIs are acquired, how signals are processed, and how other artificial intelligence technologies are combined with BCIs, such as functional electrical stimulation (FES) technology, virtual reality, exoskeletons, orthotics, and intelligent wheelchairs. We then presented the specific applications, mechanisms of action, and efficacy of BCI in the treatment of poststroke neural remodeling, such as in BCI-based neurorehabilitation of stroke patients in motor, sensory, verbal, cognitive, and environmental interactions. Finally, we summarized our recent research findings and shortcomings, as well as an outlook on the development of BCI technology in the field of rehabilitation.
2. BCI Technology
The word “brain-computer interface” was first formally identified as “a communication device that does not depend on the usual output pathways of the peripheral nerves and muscles of the brain” at the First International Conference on Brain-Computer Interface Technology in June 1999 [11]. The brain-computer interface (BCI) is a new technology that enables interaction with one’s environment through brain signals. This technology takes physiological measurements of mental states directly from the brain and converts them into control signals that can be used to control external devices or computers [12]. The BCI recognizes a set of patterns in brain signals by going through four successive stages: signal acquisition, feature extraction, feature transformation, and device output [13] (Figure 1).[…]

[Abstract] The effects of virtual reality augmented robot-assisted gait training on dual-task performance and functional measures in chronic stroke: a randomized controlled single-blind trial
Posted by Kostas Pantremenos in Gait Rehabilitation - Foot Drop, Rehabilitation robotics, Virtual reality rehabilitation on February 11, 2021
Abstract
Background
Many studies have demonstrated positive effects of virtual reality (VR) and robot-assisted gait training (RAGT) on balance, gait skills, functional capacity, active participation, and motivation in stroke patients, previously. However the effects of VR augmented RAGT on dual-task performance which requires simultaneous use of motor and cognitive parameters has not been investigated.
Aim
To primarily investigate the effects of virtual reality (VR) augmented robot-assisted gait training (RAGT) on dual-task performance and secondarily, functional measurements in chronic stroke patients.
Design
A randomized, single-blind trial.
Setting
Inpatient rehabilitation center.
Population
The study included 30 chronic stroke patients aged between 40-65 with the level of ≥ 3 from Functional Ambulation Classification and ≥ 24 from the Standardized Mini Mental State Examination.
Methods
Fifteen patients in the study group received VR augmented RAGT and 15 patients in the control group received only RAGT during 12 sessions (six weeks). All patients received neurodevelopmental therapy in addition to their treatments, simultaneously. To evaluate dualtask performance, motor and cognitive tasks were given in addition to the 10 Meter Walk (first motor task), and durations were recorded in seconds. Functional measures such as Functional Gait Assessment, Rivermead Mobility Index, Berg Balance Scale, Fall Activity Scale International, and the Functional Independence Measure for gait, mobility, balance, fear of falling, and independence in daily living activities were also applied, consecutively.
Results
The mean age of the study population was 57.93±5.91. After the treatment, single and dual-task gait speeds and cognitive dual-task performance increased in the study group (p <0.05), while no change was observed in the control group (p> 0.05). No significant difference was detected between the groups in terms of all assessments after the treatment (p>0.05).
Conclusions
This study demonstrated that VR augmented RAGT improved dual-task gait speeds and dual-task performance of chronic stroke patients however there were no difference between the two groups after the treatment. Although functional improvements were determined with VR combined RAGT approach, it was not superior to RAGT only treatment.
Clinical rehabilitation impact
The results of current study suggest the simultaneous use of VR as an adjunct therapy method to the functional training to obtain functional gains in ambulant patients with chronic stroke.
[WEB PAGE] Home-Based Rehab Program for Stroke Patients
Posted by Kostas Pantremenos in Tele/Home Rehabilitation, Video Games/Exergames, Virtual reality rehabilitation on October 22, 2020
MindMaze and Mount Sinai Health System announce the launch of an at-home tele-neurorehabilitation program for stroke patients, utilizing the FDA-cleared and CE-marked MindMotion GO.
With this system, patients can continue their recovery at home with virtual support from clinicians at Mount Sinai’s Abilities Research Center (ARC).
This initiative expands patient access to MindMotion GO, which has been adopted by the Rehabilitation Innovation team at Mount Sinai since June to provide critical neurorehabilitation across the continuum of care.

Using MindMaze’s gamified digital therapy program, Mount Sinai patients can seamlessly transition between inpatient to outpatient rehabilitation and continue their recovery at home while still receiving support and care from their physical therapists. Designed to keep acute and chronic stroke patients training for longer periods, MindMotion GO guides a complete range of body parts including the upper and lower limbs, hands, and trunk, to improve motor and task functions, a media release from MindMaze explains.
“The COVID-19 pandemic has set back the recovery and rehabilitation of stroke patients worldwide, underscoring the need for cutting-edge digital neurotherapeutics.
“MindMotion GO has enabled thousands of stroke patients to recover within the safety of their homes. We are excited to collaborate with Mount Sinai to expand access to the telerehabilitation solutions patients need and rightly deserve.”
— Tej Tadi, CEO and founder of MindMaze
MindMotion GO features several therapeutic games developed to help clinicians create personalized therapy regimens and provide patients with real-time audio and visual feedback. It also features full-body motion capture technology and hand dexterity hardware, offering therapists a way to monitor the quality of each patient’s movements and provide a tactical level of support similar to being physically present with them.
“With MindMotion GO, we’ve been able to provide our patients with continued access to top-tier telerehabilitation and support despite constant changes in the traditional hospital and treatment settings.
“We look forward to growing this program as we’ve seen our patients enjoy a new level of ownership over their treatment, helping them make great strides in their recovery.”
— David Putrino, PhD, Director of Rehabilitation Innovation for the Mount Sinai Health System
[Source(s): MindMaze, PR Newswire]
[ARTICLE] Sleep Disruption After Brain Injury Is Associated With Worse Motor Outcomes and Slower Functional Recovery – Full Text
Posted by Kostas Pantremenos in TBI on August 24, 2020
Abstract
Background. Sleep is important for consolidation of motor learning, but brain injury may affect sleep continuity and therefore rehabilitation outcomes. Objective. This study aims to assess the relationship between sleep quality and motor recovery in brain injury patients receiving inpatient rehabilitation. Methods. Fifty-nine patients with brain injury were recruited from 2 specialist inpatient rehabilitation units. Sleep quality was assessed (up to 3 times) objectively using actigraphy (7 nights) and subjectively using the Sleep Condition Indicator. Motor outcome assessments included Action Research Arm test (upper limb function), Fugl-Meyer Assessment (motor impairment), and the Rivermead Mobility Index. The Functional Independence Measure (FIM) was assessed at admission and discharge by the clinical team. Fifty-five age- and gender-matched healthy controls completed one assessment. Results. Inpatients demonstrated lower self-reported sleep quality (P < .001) and more fragmented sleep (P < .001) than controls. For inpatients, sleep fragmentation explained significant additional variance in motor outcomes, over and above that explained by admission FIM score (P < .017), such that more disrupted sleep was associated with poorer motor outcomes. Using stepwise linear regression, sleep fragmentation was the only variable found to explain variance in rate of change in FIM (R2adj = 0.12, P = .027), whereby more disrupted sleep was associated with slower recovery. Conclusions. Inpatients with brain injury demonstrate impaired sleep quality, and this is associated with poorer motor outcomes and slower functional recovery. Further investigation is needed to determine how sleep quality can be improved and whether this affects outcome.
Introduction
Sleep disturbance is a common complaint after brain injury, including stroke, with a high proportion (30%-70%) of patients presenting with impaired subjective sleep quality and meeting the criteria for at least one sleep disorder.1–4 Sleep disturbance could be resulting from direct damage to brain areas, or due to secondary effects such as being in the hospital environment, depression, anxiety or pain, and could potentially have an impact on rehabilitation through reduced engagement or impaired learning and consolidation.5
There is some evidence for improvements in sleep quality from the acute to the chronic stage of stroke6,7; however, stroke survivors at the chronic stage continue to have impaired subjective and objective sleep quality and worse quality of life than controls.8,9 Interestingly, the longer the time since stroke, the worse the perceived daytime sleepiness becomes.10 This suggests that sleep disturbance may be persistent throughout the rehabilitation period for some, and changes within this time frame in patients with different types of brain injuries are yet to be determined.
The link between sleep quality and function after stroke and brain injury is currently emerging. Siccoli et al11 demonstrated a cross-sectional correlation between the National Institute for Health Stroke Scale (NIHSS) score and wake after sleep onset (WASO), in a small sample of acute stroke patients. A larger study12 found a cross-sectional relationship between subjective sleep quality and the functional ambulation score after stroke but had no objective sleep measures. Similarly, Kalmbach et al13 found that patients with subjective difficulties initiating sleep had lower function at multiple time-points over the first 6 months of recovery from traumatic brain injury (TBI). Sleep variables, such as total sleep time, WASO and daytime napping, have also been shown to explain significant variance in Barthel Index (BI) score at the acute stage of stroke,14,15 and the percentage of sleep stages I and rapid eye movement (REM) are negatively associated with NIHSS.16
However, there is little research to indicate whether sleep quality over the rehabilitation period correlates with outcome or change in function over time, and studies that are available are somewhat inconsistent in their findings. The presence of sleep-disordered breathing at the acute stage has been found to be associated with reduced modified Rankin scale (mRS) and BI at 6 weeks poststroke17 and other studies have demonstrated that stroke patients categorized with a “poor” functional outcome have a lower sleep efficiency, less REM sleep or a reduced REM sleep latency at the acute stage than those with a better outcome.16,18,19 In contrast, Joa et al20 found no difference in the change in NIHSS or BI between patients reporting sleep disturbance at 1 month poststroke and those reporting no disturbance. They did, however, find that the group reporting no sleep disturbance had a greater improvement in the Berg Balance Scale (BBS). This was particularly evident for the moderate-severe stroke patients compared with mild (on the basis of NIHSS score at 1 week poststroke), suggesting sleep may have a greater impact on functional recovery in those who have the most relearning to achieve. The studies by Iddagoda et al4 and Joa et al20 used only subjective sleep measures and many of the studies have divided participants into groups based on outcome or the presence/absence of sleep disturbance, rather than examining both sleep quality and outcome as a continuum which may be more sensitive to differences across participants. Studies that did assess objective sleep quality as a continuum are mixed in their findings. Bakken et al15 found no correlation between sleep variables in the acute stage and BI at 6 months poststroke whereas Vock et al7 found that higher WASO or lower sleep efficiency at the acute stage poststroke was associated with worse outcome (mRS or BI score) at discharge. Similarly, Huang et al14 demonstrate that total sleep time correlates positively, and sleep latency correlates negatively, with the change in BI with rehabilitation.
As there is no clear consensus on the relationship between sleep quality measures and the rate of recovery with rehabilitation, and it is unclear how sleep quality changes over the course of rehabilitation, we sought to conduct a prospective assessment of sleep quality in neurological inpatients and explore the relationship with neurorehabilitation outcomes. We therefore assessed objective and subjective sleep quality at up to 3 time-points throughout the rehabilitation period and examined the relationship between sleep quality and motor and functional outcome measures. Specifically, we aimed to address the following questions:
- Does sleep quality at a single time-point correlate with function/impairment at that time-point?
- Does sleep quality change over the inpatient rehabilitation period?
- Does objective sleep quality averaged over the inpatient rehabilitation period explain variance in motor outcomes over that explained by baseline function?
- Does objective or subjective sleep quality averaged over the inpatient rehabilitation period explain variance in the rate of recovery in addition to covariates such as initial independence, age, and time since injury?
[…]
[Abstract] Implementing biomarkers to predict motor recovery after stroke.
Posted by Kostas Pantremenos in Paretic Hand on December 10, 2019
Abstract
BACKGROUND:
There is growing interest in using biomarkers to predict motor recovery and outcomes after stroke. The PREP2 algorithm combines clinical assessment with biomarkers in an algorithm, to predict upper limb functional outcomes for individual patients. To date, PREP2 is the first algorithm to be tested in clinical practice, and other biomarker-based algorithms are likely to follow.
PURPOSE:
This review considers how algorithms to predict motor recovery and outcomes after stroke might be implemented in clinical practice.
FINDINGS:
There are two tasks: first the prediction information needs to be obtained, and then it needs to be used. The barriers and facilitators of implementation are likely to differ for these tasks. We identify specific elements of the Consolidated Framework for Implementation Research that are relevant to each of these two tasks, using the PREP2 algorithm as an example. These include the characteristics of the predictors and algorithm, the clinical setting and its staff, and the healthcare environment.
CONCLUSIONS:
Active, theoretically underpinned implementation strategies are needed to ensure that biomarkers are successfully used in clinical practice for predicting motor outcomes after stroke, and should be considered in parallel with biomarker development.
[Thesis] Post-stroke rehabilitation of hand function based on Electromyography biofeedback – Full Text PDF
Posted by Kostas Pantremenos in Paretic Hand on April 5, 2019
Abstract
The aim of my thesis work is the application and validation of an electromyographic biofeedback (EMG-BF) system in post-stroke rehabilitation setting. The absolute number of strokes is expected to dramatically increase in coming years, thus suggesting a need for strategies to improve post-stroke assistance and rehabilitation. The electromyogram (EMG) signal has shown good perspectives in the analysis of movements and motor impairment and the introduction of closed loop rehabilitation strategies revealed an increase of patient self-consciousness and motivation. Results are promising but a lack in the optimization of the devices for the application in the clinical context has been revealed. The device and the related software employed in the present research have been specifically conceived with this purpose. The device has been optimized during a clinical pilot study and then, a complete clinical trial has been started to investigate the characteristics of post stroke patients eligible for a rehabilitation therapy with the device, and the short-term clinical effect of the therapy on the recovery of the hand functionality. A statistical analysis has been performed on the dataset collected for 3 months. The data analysis included both clinical data and data collected from patients with the device during the execution of the experimental protocol. The preliminary results of the data analysis have confirmed the suitability of the system for its intended use and highlighted that the patient ability of controlling the EMG-BF based device is related to the degree of impairment with minimum p-value<0.001, depending on the patient clinical picture and on the exercise performed.
Moreover, according preliminary results observed on four patients that received a 15 hours therapy for 3 weeks, the improvement of the parameters related to the hand and fingers motor function, suggests the efficacy of the therapy. Finally, aspects related to the analysis of continuous motions of the wrist performed during the therapy have been investigated and the relevance of the temporal information in the interpretation of this type of movements has been revealed (p<<0.01).
[Systematic Review] Trends in robot-assisted and virtual reality-assisted neuromuscular therapy: a systematic review of health-related multiplayer games – Full Text
Posted by Kostas Pantremenos in Rehabilitation robotics, Video Games/Exergames, Virtual reality rehabilitation on November 22, 2018
Abstract
Background
Multiplayer games have emerged as a promising approach to increase the motivation of patients involved in rehabilitation therapy. In this systematic review, we evaluated recent publications in health-related multiplayer games that involved patients with cognitive and/or motor impairments. The aim was to investigate the effect of multiplayer gaming on game experience and game performance in healthy and non-healthy populations in comparison to individual game play. We further discuss the publications within the context of the theory of flow and the challenge point framework.
Methods
A systematic search was conducted through EMBASE, Medline, PubMed, Cochrane, CINAHL and PsycINFO. The search was complemented by recent publications in robot-assisted multiplayer neurorehabilitation. The search was restricted to robot-assisted or virtual reality-based training.
Results
Thirteen articles met the inclusion criteria. Multiplayer modes used in health-related multiplayer games were: competitive, collaborative and co-active multiplayer modes. Multiplayer modes positively affected game experience in nine studies and game performance in six studies. Two articles reported increased game performance in single-player mode when compared to multiplayer mode.
Conclusions
The multiplayer modes of training reviewed improved game experience and game performance compared to single-player modes. However, the methods reviewed were quite heterogeneous and not exhaustive. One important take-away is that adaptation of the game conditions can individualize the difficulty of a game to a player’s skill level in competitive multiplayer games. Robotic assistance and virtual reality can enhance individualization by, for example, adapting the haptic conditions, e.g. by increasing haptic support or by providing haptic resistance. The flow theory and the challenge point framework support these results and are used in this review to frame the idea of adapting players’ game conditions.
Introduction
Robotic assistance and virtual reality in neuromuscular therapy
Neurological deficits can result in impaired motor function that affect a person’s quality of life. Researchers have been working to restore the nervous system and reduce the neurological deficits of people suffering from stroke, spinal cord injury, or traumatic brain injury [1]. For people with neurological deficits, impaired motor function is among the most prominent factors limiting the quality of life [2]. Motor neurorehabilitation can lead to permanent improvements in motor function [3]. Robotic assistance and virtual reality have the potential to enhance rehabilitation of neuromuscular deficits beyond the levels possible with conventional training strategies [4, 5].
Game experience and task performance in multiplayer games
Robot- and virtual reality-assisted single-player games are well integrated in neurorehabilitation schedules. Recently, multiplayer games have been tested to complement neuromuscular therapy. Multiplayer games are expected to motivate the patients and increase the potential of robot- and virtual reality-assisted neuromuscular therapy.
Multiplayer games incorporate social interaction to promote the enjoyment of the involved players. The additional player adds new possibilities to the game environment, generally missed in single-player gaming against preprogrammed challenges or artificially controlled opponents. The multiplayer environment and related game mechanics can facilitate social interaction, ranging from conversation to haptic interaction. Due to the this added social interaction, the game experience is thought to be better in multiplayer compared to single-player gaming [6].
The mode of the game specifies whether the players compete or cooperate with one another [7]. In line with the flow theory, a competitive mode requires opponents of similar skill level to achieve enjoyment as the task difficulty experienced by one opponent [8]. Comparable skill levels prevent boredom or stress and result in a meaningful challenge level that leads to a flow state when training [9]. In such training conditions the players have a positive game experience.
In positive game experience players increase their game performance [9, 10]. Increased game performance facilitates the general idea of serious games, i.e., playing for a primary purpose other than pure entertainment [11]. If enhanced game performance is achieved by increased physical activity, training intensity is also increased. In neuromuscular therapy, training intensity – alongside early treatment, user-centered, and task-oriented training – is one of the key factors in neurorehabilitation [12, 13]. Therefore, multiplayer gaming has great potential to further increase the benefits of robot-assisted neuromuscular and virtual reality-assisted therapy [14, 15].
[…]
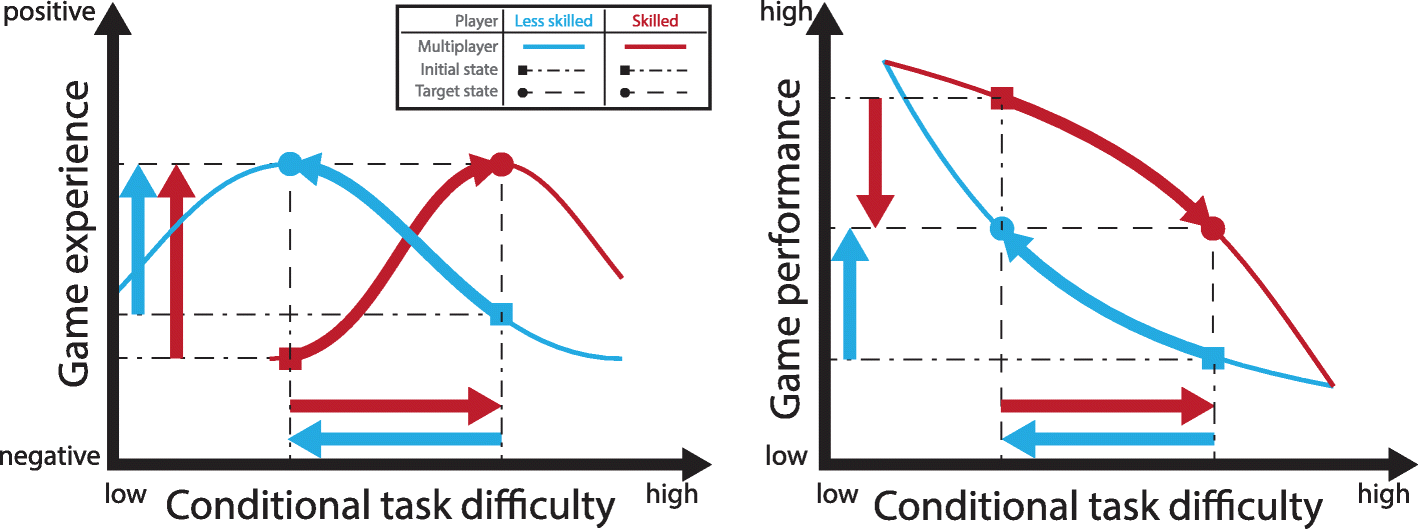
Fig. 4Difficulty adaptation based on individual condition setting in multiplayer games. Game experience (left) can be optimized by balancing the game performance (right). – Left: The initial game experience under nominal conditions relates to the skill level of the opponent and is non-optimal for differently skilled players (squares). Optimal game experience is perceived by the players when the condition adapts the difficulty towards the players’ skill level (circles). – Right: A common initial game performance state consists of a conditional task difficulty and its corresponding player specific game performance (square). Player specific difficulty adaptation can balance the game performances of the two players (circles)
