Posts Tagged neuroscience
[ARTICLE] Functional connectivity interacts with visual perceptual learning for visual field recovery in chronic stroke – Full Text
Posted by Kostas Pantremenos in Hemianopsia, Neuroplasticity on February 19, 2024
Abstract
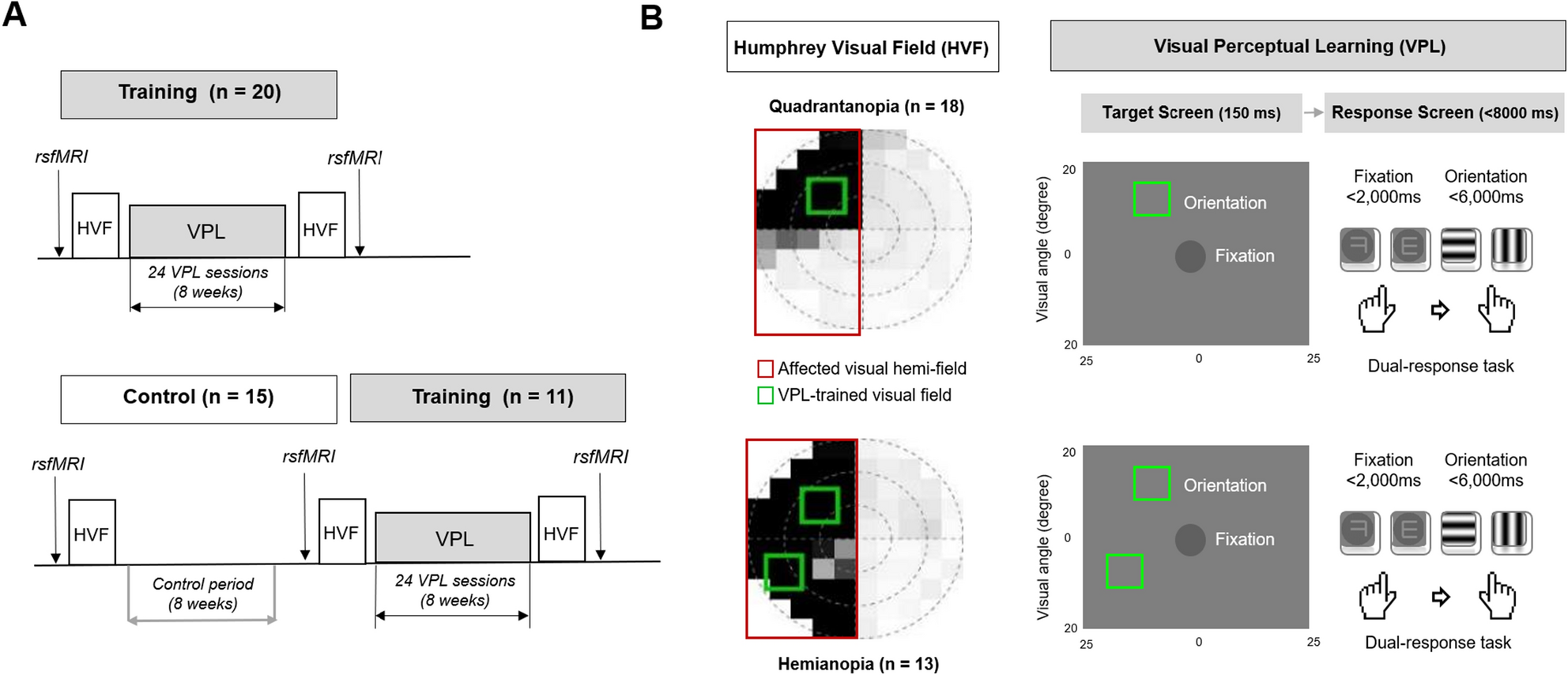
A reciprocal relationship between perceptual learning and functional brain changes towards perceptual learning effectiveness has been demonstrated previously; however, the underlying neural correlates remain unclear. Further, visual perceptual learning (VPL) is implicated in visual field defect (VFD) recovery following chronic stroke. We investigated resting-state functional connectivity (RSFC) in the visual cortices associated with mean total deviation (MTD) scores for VPL-induced VFD recovery in chronic stroke. Patients with VFD due to chronic ischemic stroke in the visual cortex received 24 VPL training sessions over 2 months, which is a dual discrimination task of orientation and letters. At baseline and two months later, the RSFC in the ipsilesional, interhemispheric, and contralesional visual cortices and MTD scores in the affected hemi-field were assessed. Interhemispheric visual RSFC at baseline showed the strongest correlation with MTD scores post-2-month VPL training. Notably, only the subgroup with high baseline interhemispheric visual RSFC showed significant VFD improvement following the VPL training. The interactions between the interhemispheric visual RSFC at baseline and VPL led to improvement in MTD scores and largely influenced the degree of VFD recovery. The interhemispheric visual RSFC at baseline could be a promising brain biomarker for the effectiveness of VPL-induced VFD recovery. […]
Figure 1
[ARTICLE] Brain oscillations in reflecting motor status and recovery induced by action observation-driven robotic hand intervention in chronic stroke – Full Text
Posted by Kostas Pantremenos in Paretic Hand, REHABILITATION, Rehabilitation robotics on December 17, 2023
Hand rehabilitation in chronic stroke remains challenging, and finding markers that could reflect motor function would help to understand and evaluate the therapy and recovery. The present study explored whether brain oscillations in different electroencephalogram (EEG) bands could indicate the motor status and recovery induced by action observation-driven brain–computer interface (AO-BCI) robotic therapy in chronic stroke. The neurophysiological data of 16 chronic stroke patients who received 20-session BCI hand training is the basis of the study presented here. Resting-state EEG was recorded during the observation of non-biological movements, while task-stage EEG was recorded during the observation of biological movements in training. The motor performance was evaluated using the Action Research Arm Test (ARAT) and upper extremity Fugl–Meyer Assessment (FMA), and significant improvements (p < 0.05) on both scales were found in patients after the intervention. Averaged EEG band power in the affected hemisphere presented negative correlations with scales pre-training; however, no significant correlations (p > 0.01) were found both in the pre-training and post-training stages. After comparing the variation of oscillations over training, we found patients with good and poor recovery presented different trends in delta, low-beta, and high-beta variations, and only patients with good recovery presented significant changes in EEG band power after training (delta band, p < 0.01). Importantly, motor improvements in ARAT correlate significantly with task EEG power changes (low-beta, c.c = 0.71, p = 0.005; high-beta, c.c = 0.71, p = 0.004) and task/rest EEG power ratio changes (delta, c.c = −0.738, p = 0.003; low-beta, c.c = 0.67, p = 0.009; high-beta, c.c = 0.839, p = 0.000). These results suggest that, in chronic stroke, EEG band power may not be a good indicator of motor status. However, ipsilesional oscillation changes in the delta and beta bands provide potential biomarkers related to the therapeutic-induced improvement of motor function in effective BCI intervention, which may be useful in understanding the brain plasticity changes and contribute to evaluating therapy and recovery in chronic-stage motor rehabilitation.
1 Introduction
Stroke has been the leading cause of acquired disability in adults globally for decades (Mendis, 2013). Although the mortality rate declined with improved healthcare, approximately 80% of stroke victims still experience motor impairment, and more than 30% of patients suffer despite intensive rehabilitation (Lai et al., 2002; Young and Forster, 2007). It is worse for the chronic group with severe motor impairments in the upper limbs. On the one hand, effective interventions like constraint-induced movement therapy (CIMT) may not be applicable to those patients without enough residual active movement (Thrasher et al., 2008). On the other hand, motor recovery in chronic stroke is more challenging due to the decreasing plasticity of spontaneous recovery (Cassidy and Cramer, 2017). Since the upper limbs, especially the hands, play a significant role in daily activity, exploring novel rehabilitation therapies for hand motor recovery in this group is essential (Neumann, 2016). Robot-assisted therapy (RAT) and motor imagery (MI) have been introduced to enhance motor recovery for stroke patients through passive motion or mental practice. However, although these interventions benefit training without requiring patients’ residual ability, rehabilitation effectiveness is still limited by a lack of active engagement (Kwakkel et al., 2008; Ietswaart et al., 2011). Recent advances in brain–computer interface (BCI) technology offer a novel method that could extract the motor intention of patients executing MI to support active rehabilitation training. Related studies have shown promising results that MI-actuated BCI improves motor ability more than pure MI or sham BCI (Ramos-Murguialday et al., 2013; Ang et al., 2014; Pichiorri et al., 2015). However, this intervention still faces limitations in practical use (Mulder, 2007; Baniqued et al., 2021). First, BCI may not be easy for everyone due to the “BCI illiteracy” phenomenon or the limited training schedule in clinical environments (Blankertz et al., 2009; Horowitz et al., 2021). In addition, most stroke subjects show more difficulty executing MI tasks than healthy subjects because of brain impairment in motor-related areas (Mulder, 2007). Worse situations occur in severe patients because they can hardly perform effective MI or fall into fatigue quickly under effortful attempts. Recent studies found that action observation (AO) could also activate sensorimotor features, as in MI and motor execution tasks (Friesen et al., 2017; Hardwick et al., 2017). In addition, repeated AO could induce plasticity changes by activating the mirror neuron system (MNS) (Rizzolatti and Sinigaglia, 2010; Agosta et al., 2017). These inspired studies combined AO in the BCI system, where stronger event-related desynchronization (ERD) responses are found than in pure MI-BCI (Kondo et al., 2015; Ono et al., 2018; Nagai and Tanaka, 2019). However, most of these studies focused on healthy subjects, while related endeavors in the clinical rehabilitation of stroke subjects are still insufficient.
Another major concern in exploring novel interventions in chronic stroke is better evaluating the motor deficits and understanding the therapeutic-induced improvement during rehabilitation neurologically. On the one hand, the recovery in post-stroke motor rehabilitation is usually heterogeneous. Except for individual factors such as age, time since stroke, and related complications, a variety of neuro-clinical factors, such as the degree of brain lesion and neural status, would also affect the patient’s recovery (Riley et al., 2011; Chang et al., 2013; Feng et al., 2015; Kim and Winstein, 2017). On the other hand, chronic stroke recovery is more challenging with the decreasing plasticity of spontaneous recovery and depends more on intervention-induced plasticity (Cassidy and Cramer, 2017). The routinely used assessment of motor recovery is on clinical scales, which are semi-objective and limited in monitoring the underlying neural factors. Hence, recent studies have focused on finding neural biomarkers that could serve as an additional physiological approach to probe brain status and reflect the extent of post-stroke functional recovery (Kim and Winstein, 2017). Potential biomarkers have been found in physiological measuring tools such as Functional magnetic resonance imaging (fMRI) and magnetoencephalograms (MEG) (Várkuti et al., 2013; Kim and Winstein, 2017).
Compared with these tools, electroencephalography (EEG) offers another economical and widely available choice, making it a more practical approach in clinical environments for rehabilitation (Gerloff et al., 2006; Ang and Guan, 2016). In addition, the EEG is easy to implement in EEG-based BCI interventions. However, most related investigations of EEG markers focused on acute or subacute-stage patients, and studies concerned with chronic patients are still lacking (Foreman and Claassen, 2012; Assenza et al., 2017; Trujillo et al., 2017; Bentes et al., 2018). Notably, EEG oscillations in different bands themselves play roles in reflecting the physiological and pathological status of the neural systems. For example, the increasing low-frequency power (delta and theta bands) and decreasing high-frequency power (alpha and beta bands) are believed to reflect the severity of acute neurological deficits (Rabiller et al., 2015; Assenza et al., 2017). Apart from reflecting the motor status, the EEG features may also promote an understanding of varied recovery resulting from additional factors during rehabilitation. For instance, a previous study found that patients under different interventions have different EEG indicators (Mane et al., 2019). We infer that patients with varying degrees of recovery may also differ in EEG features after experiencing different neural processes in training. Overall, how these EEG oscillations would act in chronic stroke and whether related EEG features could reflect therapeutic-induced improvement in effective interventions remains to be determined.
To fill this gap, the present study aimed to explore whether brain oscillations in different EEG bands can reflect the motor status and recovery induced by novel BCI therapy in chronic stroke. Specifically, an AO-BCI robotic hand training intervention was studied in a clinical environment, and the motor scales were assessed before and after the training. The correlations between EEG band power and motor scales both before and after the intervention were analyzed to study their feasibility in reflecting motor status by EEG band power in chronic stroke patients. In addition, we presented the difference in EEG variation during an intervention on patients with and without effective recovery [whether the minimal clinically important difference (MCID) was reached] (van der Lee et al., 2001; Wagner et al., 2008). Moreover, we examined which EEG rhythm variations correlate with motor function improvement and their potential as markers in reflecting therapeutic-induced neuroplasticity changes and guiding rehabilitation intervention in chronic stroke patients. […]

[WEB] Exercise Releases Chemical Signals that Boost Brain Health
Posted by Kostas Pantremenos in Neuroplasticity on December 17, 2023

Physical activity is frequently cited as a means of improving physical and mental health. Researchers at the Beckman Institute for Advanced Science and Technology have shown that exercise may also improve brain health more directly. They studied how the chemical signals released by exercising muscles promote neuronal development in the brain.
Their work appears in the journal Neuroscience.
When muscles contract during exercise, like a bicep working to lift a heavy weight, they release a variety of compounds into the bloodstream. These compounds can travel to different parts of the body, including the brain. The researchers were particularly interested in how exercise could benefit a particular part of the brain called the hippocampus.
“The hippocampus is a crucial area for learning and memory, and therefore cognitive health,” said Ki Yun Lee, a PhD student in mechanical science and engineering at the University of Illinois Urbana-Champaign and the study’s lead author. Understanding how exercise benefits the hippocampus could therefore lead to exercise-based treatments for a variety of conditions including Alzheimer’s disease.
To isolate the chemicals released by contracting muscles and test them on hippocampal neurons, the researchers collected small muscle cell samples from mice and grew them in cell culture dishes in the lab. When the muscle cells matured, they began to contract on their own, releasing their chemical signals into the cell culture.
The research team added the culture, which now contained the chemical signals from the mature muscle cells, to another culture containing hippocampal neurons and other support cells known as astrocytes. Using several measures, including immunofluorescent and calcium imaging to track cell growth and multi-electrode arrays to record neuronal electrical activity, they examined how exposure to these chemical signals affected the hippocampal cells.
The results were striking. Exposure to the chemical signals from contracting muscle cells caused hippocampal neurons to generate larger and more frequent electrical signals — a sign of robust growth and health. Within a few days, the neurons started firing these electrical signals more synchronously, suggesting that the neurons were forming a more mature network together and mimicking the organization of neurons in the brain.
However, the researchers still had questions about how these chemical signals led to growth and development of hippocampal neurons. To uncover more of the pathway linking exercise to better brain health, they next focused on the role of astrocytes in mediating this relationship.
“Astrocytes are the first responders in the brain before the compounds from muscles reach the neurons,” Lee says. Perhaps, then, they played a role in helping neurons respond to these signals.
The researchers found that removing astrocytes from the cell cultures caused the neurons to fire even more electrical signals, suggesting that without the astrocytes, the neurons continued to grow — perhaps to a point where they might become unmanageable.
“Astrocytes play a critical role in mediating the effects of exercise,” Lee says. “By regulating neuronal activity and preventing hyperexcitability of neurons, astrocytes contribute to the balance necessary for optimal brain function.”
Understanding the chemical pathway between muscle contraction and the growth and regulation of hippocampal neurons is just the first step in understanding how exercise helps improve brain health.
“Ultimately, our research may contribute to the development of more effective exercise regimens for cognitive disorders such as Alzheimer’s disease,” Lee says
[WEB] Spinal stimulation can improve arm and hand movement years after a stroke
Posted by Kostas Pantremenos in Paretic Hand on February 22, 2023

Research participant Heather Rendulic prepares to grasp and move a can of tomato soup at Rehab Neural Engineering Labs at the University of Pittsburgh.
Tim Betler/UPMC and University of Pittsburgh Schools of the Health Sciences
Pulses of electricity delivered to a precise location on the spinal cord have helped two stroke patients regain control of a disabled arm and hand, a team reports in the journal Nature Medicine.
The success should give “a lot of hope” to hundreds of thousands of people in the U.S. who’ve been disabled by a stroke, says Dr. Walter Koroshetz, director of the National Institute of Neurological Disorders and Stroke, which helped fund the research.
The results will need to be replicated in a larger study, Koroshetz says, adding that it’s still unclear which stroke patients will benefit most from the treatment.
For Heather Rendulic, 33, one of the patients in the study, the treatment was life-changing.

The medical team at UPMC Presbyterian hospital prepares Rendulic for the implantation of the spinal cord stimulation electrodes.
Tim Betler/UPMC and University of Pittsburgh Schools of the Health Sciences
As a teenager, Rendulic liked to run and ride horses. Then, beginning in 2011, she had a series of strokes caused by malformed blood vessels in her brain. The last stroke was the worst.
“I woke up and I couldn’t move the whole left side of my body,” Rendulic says.
Surgeons were able to remove the cluster of blood vessels that had caused her strokes. But the damage was done.
By subscribing, you agree to NPR’s terms of use and privacy policy. NPR may share your name and email address with your NPR station. See Details. This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
“It took me almost two years to walk on my own unassisted,” says Rendulic, who wrote a book about her experiences.
Rendulic was eventually able to move her arm and hand a bit. For example, she could close her hand, but not open it. As a result, she was unable to tie her own shoes, open a jar, or chop vegetables.

University of Pittsburgh neurosurgeon Dr. Peter Gerszten (left) and assistant professor of neurosurgery Marco Capogrosso, during the implantation procedure at UPMC Presbyterian hospital.
Tim Betler/UPMC and University of Pittsburgh Schools of the Health Sciences
“You don’t realize how many things you need two hands for until you only have one good one,” she says.
So nearly a decade after her strokes, Rendulic volunteered for a study at the University of Pittsburgh.
Researchers there knew that in most people like Rendulic, the brain is still trying to send signals through the spine to the muscles that control the arm and hand. Marco Capogrosso, an assistant professor in the department of neurosurgery, says the problem is that those signals are very weak.

University of Pittsburgh kinematic occupational therapist Amy Boos (left) and Carnegie Mellon University graduate student Nikhil Verma (middle) connect muscle activation sensors on Rendulic.
Tim Betler/UPMC and University of Pittsburgh Schools of the Health Sciences
“We wanted to pick up on these weak signals and essentially turn them into functional outputs so that a person would be able to control their own hand voluntarily,” he says.
Capogrosso and a team of researchers hoped to do this by delivering pulses of electricity to nerve cells in the spine. The electricity makes these nerve cells more responsive, or excitable, which helps signals from the brain get through to the muscles they control.

(Left) A close-up of a stimulating electrode containing eight stimulation contacts. (Right) Gerszten explains the placement of stimulating electrodes while holding one in his hand.
Tim Betler/UPMC and University of Pittsburgh Schools of the Health Sciences
When the team tried this in animals, they were able to restore arm and hand function.
“If you carefully place the electrodes inside the spinal cord, you can direct this excitability toward the muscles you need,” Capogrosso says.
The team was pretty sure their approach would work in people, he says. “But we didn’t expect the amount of movement recovery that we observed.”

University of Pittsburgh graduate student Erynn Sorensen (left) observes research participant Rendulic during the isometric torque test used to measure arm strength.
Tim Betler/UPMC and University of Pittsburgh Schools of the Health Sciences
Rendulic was the first person they treated. A surgeon used a large needle to place the electrodes in her spine. “I had wires hanging out of my back,” she says.
Later, in the lab, researchers turned on the stimulation. The effect was immediate.
“I was opening my hand in ways that I haven’t in ten years and my husband and my mom were with us and we all were in tears,” Rendulic says.

Graduate students (foreground) observe a testing procedure.
Tim Betler/UPMC and University of Pittsburgh Schools of the Health Sciences
The difference is easy to see in a video made by the researchers that shows Rendulic trying to pick up a can of soup.
At first, “you can see she can’t really do anything with her hand,” says Elvira Pirondini, a research assistant professor in physical medicine and rehabilitation. “But when the stimulation is on she can reach the soup and she can grab the can and also elevate it.”
Marc Powell et al YouTube
The electrical pulses also improved something many stroke patients lose — the ability to sense the position of her arm and hand without looking at them, which comes from a sort of sixth sense known as “proprioception.”
“When the stimulation was on, it was much easier for her to understand where her arm was in space.” Pirondini says.
Rendulic gives a thumbs up while holding a fork with a piece of steak with her affected arm.
Tim Betler/UPMC and University of Pittsburgh Schools of the Health Sciences
The effects of stimulation became more dramatic during the four weeks each patient had the electrodes in their spine.
“They start by opening the hand and by the end of the four weeks they can do all sorts of things,” Capogrosso says.
Also, the effects diminished but did not disappear entirely when the stimulation was switched off. That suggests the pulses are causing changes to the circuits controlling the arm and hand, Capogrosso says, though it’s not clear how long those changes will last.
At the end of the four-week study, the electrodes were removed from both patients. But researchers say they plan to develop a system that can be implanted permanently.
Ordinarily, moving this sort of technology from the lab to widespread use takes many years. But the process is likely to move much faster in this case because the device used to stimulate the spine is already approved by the Food and Drug Administration for treating patients with chronic pain.
“There are thousands of patients implanted with this technology,” Pirondini says.
Spinal stimulation has also been used to help patients paralyzed by a spinal injury regain the ability to walk.
“I don’t see any deal breakers on the way of getting this to [stroke] patients,” Koroshetz says.
Rendulic says her experience has changed the way she views her future, and she hopes to be first in line to receive a permanently implanted stimulator.
[BLOG POST] 7 principles of neuroscience every coach and therapist should know
Posted by Kostas Pantremenos in Neuroplasticity on December 3, 2022
What does neuroscience have to do with coaching and therapy?
Short answer: Quite a lot!
If you’re a coach or therapist, your job is to facilitate change in your client’s
- thinking (beliefs and attitudes)
- emotions (more mindfulness and resilience)
- behaviour (new healthy habits).
Coaching builds the mental skills needed to support lasting change. Skills such as:
- mindfulness
- self-awareness
- motivation
- resilience
- optimism
- critical thinking
- stress management
Health and wellness coaching, in particular, are emerging as powerful interventions to help people initiate and maintain sustainable change.
And we have academic research to support this claim: check out a list of RCTs in table 2 of this paper).
How can neuroscience more deeply inform coaching and therapy?
Back in the mid-1990s when I was an undergrad, the core text of my neuroscience curriculum was ‘Principles of Neural Science’ by Eric Kandel, James Schwartz and Thomas Jessell. Kandel won the 2000 Nobel Prize in Physiology or Medicine for his research on memory storage in neurons.
A few years before his Nobel, Kandel wrote a paper ‘A new intellectual framework for psychiatry’. The paper explained how neuroscience can provide a new view of mental health and wellbeing.
Based on Kandel’s paper, researchers at the Yale School of Medicine proposed seven principles of brain-based therapy for psychiatrists, psychologists and therapists. The principles have been translated into practical applications for health & wellness, business, and life coaches.
One fundamental principle is,
“All mental processes, even the most complex psychological processes, derive from the operation of the brain.”
And another is:
“Insofar as psychotherapy or counseling is effective . . . it presumably does so through learning, by producing changes in gene expression that alter the strength of synaptic connections.”
That is, human interactions and experience influence how the brain works.
This concept of brain change is now well established in neuroscience and is often referred to as neuroplasticity. Ample neuroscience research supports the idea that our brains remain adaptable (or plastic) throughout our lifespan.
Here is a summary of Kandel, Cappas and colleagues thoughts on how neuroscience can be applied to therapy and coaching…
Seven principles of neuroscience every coach should know.
1. Both nature and nurture win.
Both genetics and the environment interact in the brain to shape our brains and influence behaviour.
Therapy or coaching can be thought of as a strategic and purposeful ‘environmental tool’ to facilitate change and may be an effective means of shaping neural pathways.
2. Experiences transform the brain.
The networks of our brain associated with emotions and memories such as the pre-frontal cortex, the amygdala, and the hippocampus are not hard-wired — they are ‘plastic’. The brain prunes and tunes its connections in response to the experiences it has.
3. Memories are imperfect.
Our memories are never a perfect account of what happened. Memories are re-written each time when we recall them depending on how, when and where we retrieve the memory.
For example, a question, photograph or a particular scent can interact with a memory resulting in it being modified as it is recalled.
With increasing life experience we weave narratives into their memories. Autobiographical memories that tell the story of our lives are always undergoing revision precisely because our sense of self is too.
Consciously or not, we use imagination to reinvent our past, and with it, our present and future.
4. Emotion underlies memory formation.
Memories and emotions are interconnected neural processes.
The amygdala, which plays a role in emotional arousal, mediate neurotransmitters essential for memory consolidation. Emotional arousal has the capacity to activate the amygdala, which in turn modulates the storage of memory.
Research suggests each of us constructs emotions from a diversity of sources: our physiological state, by our reactions to the ‘outside’ environment, experiences and learning, and our culture and upbringing.
5. Relationships are the foundation for change
Relationships in childhood AND adulthood have the power to elicit positive change.
Sometimes it takes the love, care or attention of just one person to help another change for the better.
The therapeutic relationship has the capacity to help clients modify neural systems and enhance emotional regulation.
6. Imagining and doing are pretty much the same thing to the brain.
Mental imagery or visualisation not only activates the same brain regions as the actual behaviour but also can speed up the learning of a new skill.
Envisioning a different life may as successfully invoke change as the actual experience.
7. We don’t always know what our brain is ‘thinking’.
Unconscious processes exert great influence on our thoughts, feelings, and actions (but I’m not willing to put a percentage on ‘how much is subconscious’).
The brain can process nonverbal and unconscious information, and such information influences therapeutic and other relationships. It’s possible to react to unconscious perceptions without consciously understanding the reaction.
[WEB] Neurology, Neuroscience & Neurosurgery websites – UCL
Posted by Kostas Pantremenos in Neuroplasticity, resources on November 30, 2022

Neurology, Neuroscience & Neurosurgery websites
- Neuro websites
- Neuro gateways
- Neuro organisations
- Neuro history
- Neuro people
- Neuro images
- Medical terminology
Neuro-websites
- Allen Brain Atlas (ABA) – A growing collection of online public resources integrating extensive gene expression and neuroanatomical data, complete with a suite of search and viewing tools.
- Biomed central: neuroscience, neurology and psychiatry gateway – links to selected Biomed Central open access journals together with links to other subject-related resources.
- Brain Charity is a unique non-medical advice and information centre for people with neurological illnesses and those who care for them.
- Cambridge Neuroscience (UK) Information about research activities, news and events at the Cambridge research centre.
- Child neurology online The main purpose of this site is to coordinate the available internet resources in Child Neurology, both for professionals and patients.
- CNS Pathology Index. (USA) A web page with links to full colour images of the brain.
- Dana Brainweb (USA) Information about common brain diseases and disorders and the internet’s best general neuroscience resources.
- George Washington University Hospital Comprehensive Stroke Center (Washington, USA). Up-to-date resources and protocols
- Martindale’s Health Science Guide ’04 (USA) includes sections on: Anaesthesiology and Surgery virtual medical Centre contains section on Neurosurgery; Anatomy and Histology Centre: Brain stem section; Anatomy and Histology Centre: Nervous System Section; Otolaryngology, Otorhinolaryngology & Ophthalmology Centre.
- MIT OpenCourseWare: brain and cognitive sciences (USA) – free access to lecture notes from over 100 of undergraduate and graduate courses from MIT’s Department of Brain & Cognitive Sciences.
- Multiple Sclerosis (US) – an introduction to multiple sclerosis for medical students and physicians in training.
- National Society for Epilepsy “The Society runs in conjunction with the National Hospital for Neurology and Neurosurgery an inpatient epilepsy treatment centre and epilepsy outpatients clinic. It organises respite care and sheltered workshops. It provides information and an education service. It has a network of self help groups providing information and support to individuals and their families in their own locality.”
- Neuromuscular Disease (TREAT-NMD network). An international initiative bringing together over 300 doctors, researchers and other professionals throughout 11 European countries. EU-funded. Aims to improve treatment, share good practice and improve global standards of care.
- Neuromuscular Disease Center (USA). Created by Washington University, MO, this is a very detailed list of resources devoted to neuromuscular diseases.
- Neuro-ophthalmology atlas (USA) Endorsed by the International Council of Ophthalmology, “The Atlas of Ophthalmology is a public online database, free of charge, edited by specialists in the field”.
- NHS Inform: brain, nerves and spinal cord NHS inform is Scotland’s national health information service, with the aim of providing the people in Scotland with accurate and relevant information to help them make informed decisions about their own health and the health of the people they care for.
- NICE Guidance: neurological conditions Links to guidelines, pathways and quality standards for a number of neurological conditions.
- Royal College of Nursing elearning Developed with the Motor Neurone Disease Association, this resource is aimed at nurses and student nurses, and covers symptoms and diagnosis, and caring for someone with motor neurone disease.
- Royal College of Physicians Stroke Programme The CEEU Stroke Programme at the Royal College of Physicians is guided by the Intercollegiate Working Party for Stroke, which is made up of representatives of all the organisations which support the different disciplines involved in the management of stroke, including patients. The programme aims to improve the care of stroke across the country. It includes the development, up-dating and co-ordination of guidelines, patient information, and clinical audit.
- Statista – a statistical website offering stats around dementia and related topics.
- Whole Brain Atlas (USA)
Neuro-gateways
- Neurosciences on the Internet
- Neuroscience Resources from Eric H. Chudler’s Systems (USA)
- Neurosciences Virtual Library (USA)
- Neuropsychology Central Directory devoted exclusively to the subject of Human Neuropsychology, although several links are out of date.
Neuro-organisations
- American Association of Neurological Surgeons
- American Academy of Neurology
- Association of British Neurologists (ABN) “the organisation that represents clinical and academic neurologists in the United Kingdom.” The ABN promotes education of neurological trainees, and neurological learning throughout medical training. Resources include ABN statements, guidelines, commissioning toolkits and quality standards.
- British Neuropsychiatry Association (BNPA) “the principal academic and professional body for medical practitioners and professionals allied to medicine working at the interface of the clinical and cognitive neurosciences, and psychiatry”.
- European Federation of Neurological Societies
- Health Careers: Neurology – information about the specialty itself, training and common procedures and interventions.
- International Society of Neuropathology
- Joint Royal Colleges of Physicians Training Board – information about specialty training.
- National Institute of Neurological Disorders and Stroke (NINDS) (US) – an excellent source of factsheets on neurological disorders
- World Muscle Society (WMS) “an international, multidisciplinary, scientific society, dedicated to the advancement and dissemination of knowledge in the field of neuromuscular disorders, for the benefit of patients.”
Neuro-history
- Cyber Museum of Neurosurgery (USA)
- History of Medicine – an online version of the classic annotated bibliography commonly known as “Garrison-Morton”, listing classic publications for many areas of medicine including neuro-topics, many pre-date the coverage of search sources such as Pubmed.
- History of Neurosurgery at Massachusetts General Hospital & Harvard Medical School (USA)
- History of Neuroscience by Eric H. Chudler, Ph.D., Research Assistant Professor, Dept. of Anesthesiology, University of Washington
- Harvey Cushing/John Hay Whitney Medical Library Yale University (USA)
- Images from the History of Medicine (USA) – a searchable collection of about 100,000 images from the prints and photograph collection of the National Library of Medicine.
- ECHO (Exploring and Collecting History Online) from the Center for History and New Media, George Mason University. Database concerned with the history of science, technology and industry.
- Whonamedit.com is a biographical dictionary of medical eponyms and is also useful for finding bibliographical dates
- Nobel Prize – more than 7000 documents about the Nobel prizes. Including portraits and biographies of laureates. Includes Carlsson, Greengard & Kandel, Prize for Physiology or Medicine 2000 “for their discoveries concerning signal transduction in the nervous system”.
- Wellcome Trust (UK) – History of Medical Collection: a free catalogue of evaluated, high quality Internet resources and websites relating to the history of medicine and allied sciences, covering all aspects of the history of health and development of medical knowledge.
Neuro-people
Including online directories of neurologists, neurosurgeons & neuroscientists:
- UK – specialistinfo.com a directory of UK consultants (requires free registration)
- US – American Association of Neurological Surgeons (AANS) including the Find a Neurosurgeon service
- US – American Academy of Neurology (AAN)
- Worldwide – World Directory of Neurological Surgeons
Neuro-images
- anatomy tv – an interactive 3D human anatomy for UCL users. No login is required if you access the service via the Primal Pictures link while connected to the UCL network (or access via Desktop@UCL Anywhere when working offsite). Unfortunately NHS access to this resource has ceased, as NHS funding was no longer available to support this subscription.
- flickr – photo/image-sharing site with many images freely available for re-use through Creative Commons arrangements.
- HONmedia – a repository of medical images and videos covering 1,700 topics and themes.
- NHS Scotland Photo Library – a resource for the NHS providing photography for use in communication materials.
- University of Iowa Digital Library – includes Charles Bell’s classic images of trepanning.
- US NLM Images from the History of Medicine. searchable collection of over 65,000 images.
- Visible Body (Argosy) – “the most comprehensive human anatomy visualization tool available” 3D models of over 1,700 anatomical structures, including all major organs and systems of the human body. Compatible with Internet Explorer.
- Wellcome Collection YouTube Channel (UK) – videos relating to medical history from the Wellcome Collection.
Medical Terminoloy
- eMedicine Dictionary
- Medical terminology eLearning tool (NHS UK)
- Stedman’s Medical Dictionary
- TheFreeDictionary (medical section)
[WEB] New FDA-Approved Device for Stroke Rehabilitation Now Available for Patients at Jefferson Health
Posted by Kostas Pantremenos in Neuroplasticity, Paretic Hand, Recovery Plateau on November 18, 2022
The device improves upper limb function for patients with disability after stroke by stimulating the vagus nerve during rehab training.
by Thomas Jefferson University
Newswise — PHILADELPHIA –Stroke affects nearly 14 million people worldwide per year, and can frequently produce loss of mobility. Patients can regain some function with intensive rehabilitation therapy for the first few months after stroke, but this recovery often plateaus and patients are left with a ‘new normal.’ To combat this plateau, Jefferson Health surgeons recently became the 5th center in the United States to implant a new FDA-approved device that improves the effectiveness of rehabilitation therapy and helps restart recovery to improve a stroke survivor’s upper mobility.
“For some patients post-stroke, paired vagus nerve stimulation may offer a path to a better quality of life and greater independence,” says Robert Rosenwasser, MD, president of the Vickie and Jack Farber Institute for Neuroscience – Jefferson Health, and Osterholm Professor and Chair of the Department of Neurological Surgery at Thomas Jefferson University. “Jefferson is leading the charge in offering patients access to the best care and most effective medical technology at all stages of stroke care and recovery.”
The device, called Vivistim, is placed under the skin in the upper chest and stimulates the vagus nerve in the neck, which sends signals to the brain leading to additional release of neurotransmitters. Vagus nerve stimulation (VNS) is an extensively researched option for several neurological conditions and is also FDA approved to treat specific cases of depression and epilepsy. The results of a triple-blinded, randomized controlled clinical trial published in The Lancet, show that pairing VNS therapy with post-operative rehabilitation can generate two to three times more hand and arm function for stroke survivors than rehabilitation therapy alone. Mobility and control improved even in patients who were several years out from their stroke, at a time when little improved mobility is expected from additional rehabilitation.
The first Jefferson patient, who suffered a stroke two years ago, had surgery with Dr. Reid Gooch, Assistant Professor of Neurosurgery, in late October and recently began rehabilitation with a dedicated team of neurological occupational therapists at MossRehab.
“Jefferson is exploring many avenues to help restore post-stroke patients’ daily function and abilities,” says neurologist Mijail Serruya, MD, PhD, co-director of the Center for Neurorestoration at the Farber Institute for Neuroscience. “Technologies that improve or augment the connection between brain and paralyzed parts of the body are promising. We seek to make innovative treatments – whether developed in industry, or here in our new Center, available to improve outcomes for patients.”
During rehabilitation therapy, the device is activated to give a gentle pulse to the vagus nerve while the patient performs a specific task, such as reaching for a cup, or cutting food. The stimulation helps strengthen the automatic learning pathways so that these actions become easier to perform. After in-clinic Paired VNS Therapy is initiated, Vivistim can be used by the patient at home as directed by their rehabilitation specialist.
“Rehabilitation therapy is an essential component of stroke recovery,” says Alberto Esquenazi, MD, CMO of MossRehab and now part of Jefferson Health. “Together, with our extremely hard-working patients, we can help reestablish the connections between brain and limb, ultimately helping patients improve function. Technologies that help patients gain improved function are incredibly valuable.”
“We applaud the teams at Jefferson Health and MossRehab for envisioning Vivistim System as part of the complete care that is offered by their comprehensive stroke center to improve stroke survivors’ independence and quality of life,” says Richard Foust, CEO of MicroTransponder, a medical device company that develops solutions to restore independence and dignity for people suffering from neurological conditions that impair sensory and motor function. “Paired VNS Therapy addresses an unmet need for those who have chronic impairment after a stroke, so it’s a significant milestone for survivors that Vivistim programs are being built here and across the country.”
Involved in this Initiative: Neurosurgery: Robert Rosenwasser, Reid Gooch, Pascal Jabbour, Stavrapoula Tjoumakaris, Chengyuan Wu, Nabeel Herial, Nancy Tworek; Neurology: Robin Dharia, Diana Tzeng, Elan Miller, Shaista Alam, Lisa Bowman, Rodney Bell; Rehabilitation: Steve Williams , Alberto Esquenazi, Davis Berzin,; Center for Neurorestoration: Mijail Serruya, Ashwini Sharan, Joe Kardine, Dana Johnson, Gabrielle Carpino , Erica Jones, Alessandro Napoli, Phyo Thuta Aung, Rachel Zarin, Nabila Shawki, Daniel Verbit, Michelle Mattera Keon.
[ARTICLE] Long-term effect of additional rehabilitation following botulinum toxin-A on upper limb activity in chronic stroke: the InTENSE randomised trial – Full Text
Posted by Kostas Pantremenos in Pharmacological, Spasticity on October 15, 2022
Abstract
Background
It is common for people with persistent spasticity due to a stroke to receive an injection of botulinum toxin-A in the upper limb, however post-injection intervention varies.
Aim
To determine the long-term effect of additional upper limb rehabilitation following botulinum toxin-A in chronic stroke.
Method
An analysis of long-term outcomes from national, multicenter, Phase III randomised trial with concealed allocation, blinded measurement and intention-to-treat analysis was carried out. Participants were 140 stroke survivors who were scheduled to receive botulinum toxin-A in any muscle(s) that cross the wrist because of moderate to severe spasticity after a stroke greater than 3 months ago, who had completed formal rehabilitation and had no significant cognitive impairment. Experimental group received botulinum toxin-A plus 3 months of evidence-based movement training while the control group received botulinum toxin-A plus a handout of exercises. Primary outcomes were goal attainment (Goal Attainment Scale) and upper limb activity (Box and Block Test) at 12 months (ie, 9 months beyond the intervention). Secondary outcomes were spasticity, range of motion, strength, pain, burden of care, and health-related quality of life.
Results
By 12 months, the experimental group scored the same as the control group on the Goal Attainment Scale (MD 0 T-score, 95% CI -5 to 5) and on the Box and Block Test (MD 0.01 blocks/s, 95% CI -0.01 to 0.03). There were no differences between groups on any secondary outcome.
Conclusion
Additional intensive upper limb rehabilitation following botulinum toxin-A in chronic stroke survivors with a disabled upper limb is not more effective in the long-term.
Backgroud
Stroke represents a huge burden on the health care system. A meta-analysis has shown that botulinum toxin-A injections reduce spasticity compared to placebo [1], but that this reduction in spasticity does not carry over to an improvement in the ability to perform everyday activities [2, 3]. After formal rehabilitation ceases, it is common for people with persistent spasticity due to their stroke to attend a ‘Spasticity Clinic’ where they may receive an injection of botulinum toxin-A in the upper limb, particularly into muscles of the forearm and hand [4, 5]. Thereafter, post-injection intervention varies widely due to a lack of evidence, with around a third of Australian clinics only providing handouts or advice to encourage motor training [5] in the absence of supervised therapy. Therefore, we designed an intensive upper limb rehabilitation program based on evidence-based guidelines for stroke that was to be provided post-injection. The three-month program – InTENSE – included 2 weeks of serial casting aimed at decreasing any contracture [6] that was then followed by 10 weeks of movement training, aimed at decreasing weakness [7] and improving movement [8, 9]. The program was designed to be patient driven; it was mostly carried out at home supported by phone calls, home visits and occasional attendance at the clinic. We then conducted a Phase III randomised trial to determine the clinical effect of additional upper limb rehabilitation following botulinum toxin-A [10]. The findings suggested that, in stroke survivors attending a spasticity clinic who were scheduled to receive botulinum toxin-A to a muscle crossing the wrist, an additional 3 months of evidence-based movement training was no more effective than botulinum toxin-A plus usual care in terms of goal attainment and upper limb activity. We concluded that in chronic, severely disabled stroke survivors, it is not worthwhile spending resources on providing anything more than usual care after botulinum toxin-A. This paper presents the long-term outcomes of this Phase III clinical trial in order to see if anything had changed.[…]
[Abstract] Post-traumatic stress disorder: clinical and translational neuroscience from cells to circuits
Posted by Kostas Pantremenos in REHABILITATION on May 12, 2022
Abstract
Post-traumatic stress disorder (PTSD) is a maladaptive and debilitating psychiatric disorder, characterized by re-experiencing, avoidance, negative emotions and thoughts, and hyperarousal in the months and years following exposure to severe trauma. PTSD has a prevalence of approximately 6–8% in the general population, although this can increase to 25% among groups who have experienced severe psychological trauma, such as combat veterans, refugees and victims of assault. The risk of developing PTSD in the aftermath of severe trauma is determined by multiple factors, including genetics — at least 30–40% of the risk of PTSD is heritable — and past history, for example, prior adult and childhood trauma. Many of the primary symptoms of PTSD, including hyperarousal and sleep dysregulation, are increasingly understood through translational neuroscience. In addition, a large amount of evidence suggests that PTSD can be viewed, at least in part, as a disorder that involves dysregulation of normal fear processes. The neural circuitry underlying fear and threat-related behaviour and learning in mammals, including the amygdala–hippocampus–medial prefrontal cortex circuit, is among the most well-understood in behavioural neuroscience. Furthermore, the study of threat-responding and its underlying circuitry has led to rapid progress in understanding learning and memory processes. By combining molecular–genetic approaches with a translational, mechanistic knowledge of fear circuitry, transformational advances in the conceptual framework, diagnosis and treatment of PTSD are possible. In this Review, we describe the clinical features and current treatments for PTSD, examine the neurobiology of symptom domains, highlight genomic advances and discuss translational approaches to understanding mechanisms and identifying new treatments and interventions for this devastating syndrome.
Key points
- Post-traumatic stress disorder (PTSD) is a debilitating neuropsychiatric disorder, characterized by re-experiencing, avoidance, negative emotions and thoughts, and hyperarousal.
- PTSD is frequently comorbid with neurological conditions such as traumatic brain injury, post-traumatic epilepsy and chronic headaches.
- PTSD has a prevalence of approximately 6–8% in the general population and up to 25% among individuals who have experienced severe trauma.
- Many of the neural circuit mechanisms that underlie the PTSD symptoms of fear-related and threat-related behaviour, hyperarousal and sleep dysregulation are becoming increasingly clear.
- Key brain regions involved in PTSD include the amygdala–hippocampus–prefrontal cortex circuit, which is among the most well-understood networks in behavioural neuroscience.
- Combining molecular–genetic approaches with a mechanistic knowledge of fear circuitry will enable transformational advances in the conceptual framework, diagnosis and treatment of PTSD.
