Posts Tagged Hemiparetic
[Abstract] Increasing Hand Opening in Chronic Hemiparetic Stroke Using Nerve Blocks – Research Poster
Posted by Kostas Pantremenos in Paretic Hand on December 17, 2022
Research Objectives
We investigated the best-case efficacy of emerging nerve block technologies in improving hand opening in individuals with stroke. Upper limb disability in chronic stroke stems from significant flexor bias and weakness of extensors, with hand opening particularly compromised in moderate to severely impaired individuals with stroke. A complete motor block of the ulnar and median nerves may improve volitional hand opening and/or hand opening with assistive Functional Electrical Stimulation (FES).
Design
A single participant case study, quantifying hand opening before and after administration of the local anesthetic ropivacaine to the median and ulnar nerve.
Setting
Academic research laboratory.
Participants
A 63-year-old male with a unilateral hemorrhagic stroke (> 2 years).
Interventions
The participant’s forearm was attached to a robotic device capable of providing support or applying a load, and their fingers were instrumented with motion-tracking sensors. An FES unit was attached to forearm extensors. We asked the participant to perform multiple hand opening tasks, with the forearm fully supported or under a load (30% of maximum shoulder abduction), and then either volitionally or by FES. These tasks were then undergone again 1.5 hours after the complete nerve block just above the elbow.
Main Outcome Measures
The primary outcome metric is Hand Pentagonal Area (HPA), which is the surface area (mm2) measured by 3 triangles: thumb, index, middle; thumb, middle, ring; and thumb, ring, and pinky.
Results
Mean volitional hand opening in the supported condition increased significantly (two-sample t-test p≪0.001) from 1311.7mm2 to 2944.2mm2, and significantly (p≪0.001) in the loaded condition from 1383.3mm2 to 2387.7mm2. Mean FES hand opening in the supported condition increased (no significance) from 1976.1mm2 to 2177.8mm2 and in the loaded condition significantly (p=0.0047) from 1832.7mm2 to 2865.8mm2.
Conclusions
These preliminary results indicate that a complete motor block of the median and ulnar nerve can improve both the volitional and assistive FES hand opening area in an individual with chronic stroke. Emerging instant and reversible nerve block technologies may thus provide potential benefit to this population.
[ARTICLE] Upper-Limb Robot-Assisted Therapy Based on Visual Error Augmentation in Virtual Reality for Motor Recovery and Kinematics after Chronic Hemiparetic Stroke: A Feasibility Study – Full Text
Posted by Kostas Pantremenos in Paretic Hand, Rehabilitation robotics, Virtual reality rehabilitation on July 1, 2022
Abstract
The purpose of this study was to investigate the effect of upper-limb robot-assisted therapy based on visual error augmentation in virtual reality (UL-RAT-VEAVR) for motor recovery and kinematics after chronic hemiparetic stroke. This study applied a single-group pre- and post-intervention study design. A total of 27 stroke survivors (20 males and 7 females; mean age 54.51 years, mean onset duration 12.7 months) volunteered to participate in this study. UL-RAT-VEAVR was performed three times a week for four weeks, amounting to a total of twelve sessions, in which an end-effector-based robotic arm was used with a visual display environment in virtual reality. Each subject performed a total of 480 point-to-point movements toward 3 direction targets (medial, ipsilateral, and contralateral side) in the visual display environment system while holding the handle of the end-effector-based robotic arm. The visual error (distance to the targets on the monitor) in virtual reality was increased by 5% every week based on the subject’s maximum point-to-point reaching trajectory. Upper-limb motor recovery was measured in all subjects using the Fugl–Meyer Assessment (FMA) upper-limb subscale, the Box and Block Test (BBT), and the Action Research Arm Test (ARAT), before and after training. In addition, a kinematic assessment was also performed before and after training and consisted of time, speed, distance, and curvilinear ratio for point-to-point movement. There were significant improvements in both upper-limb motor function and kinematics after 4 weeks of UL-RAT-VEAVR (p < 0.05). Our results showed that the UL-RAT-VEAVR may have the potential to be used as one of the upper-limb rehabilitation strategies in chronic stroke survivors. Future studies should investigate the clinical effects of the error-augmentation paradigm using an RCT design.
1. Introduction
About 15 million people worldwide experience a stroke every year [1], resulting in various motor impairments, such as limitation of the range of motion, errors in arm movement, and abnormal joint coupling [2]. Motor impairment is one of the most common problems after stroke, and the recovery of upper extremity function is a priority for stroke survivors [3]. Upper extremity motor impairments, which require a high level of fine motor control of the arms and hands, are more likely to result in activity restriction than lower-extremity motor impairments [4]. Although recovery of upper-extremity motor function is essential for stroke patients to take care of themselves and perform activities of daily life (ADLs) [5], only 35–70% of stroke survivors recover functional levels of arm movement, and more than 50% have persistent upper extremity deficits [4,6].
In recent years, there has been a growing trend toward using interactive technology for the restoration of upper-extremity motor control in stroke survivors [7]. Robot-assisted therapy is an advanced technology that is increasingly used in post-stroke upper-extremity rehabilitation [8]. The use of robotics in stroke rehabilitation can provide high convenience, compared with traditional approaches, when performing task-oriented training, and it can provide the advantage of increasing the accuracy of measurement of kinematic results, such as movement speed and trajectory straightness [9]. Generally, upper-limb rehabilitation robots are categorized into the end-effector and exoskeleton types, according to their mechanical structure [10]. The end-effector models are connected to the user at one distal point to allow the reproduction of the dynamic environment that corresponds to ADLs, whereas the exoskeleton models can train specific muscles by controlling joint motion with calculated torque at multiple points [10,11]. In rehabilitation strategies employing the use of robotics, a key factor influencing the recovery of motor control is feedback, which is information provided through an individual’s performance results [4]. The feedback in robotic rehabilitation can be used as a source for providing knowledge about the results of movement performance, and it has been proposed as means to promote motor learning and improve motor performance through two main paradigms—error reduction (ER) and error augmentation (EA) [12].
The ER paradigm, also known as haptic guidance, is based on the hypothesis that, by guiding the subject to the correct movement trajectory, motor learning can be induced through imitation [4,13]. In other words, the ER paradigm aims to reduce a subject’s movement errors during motor performance. In contrast, the EA paradigm uses visual or sensory feedback to magnify the error along the desired trajectory [14]. The ER paradigm is often seen as counterintuitive because it contrasts with conventional approaches that aim to minimize patient movement errors. However, iterative learning of EA has shown the potential to promote movement control [15,16]. In addition, EA learning is considered a major factor in neuroplasticity and the reacquisition of movement skills [16,17]. A previous systematic review [4] suggested that robotic therapy using the EA paradigm is more effective than conventional repetitive practice for upper-extremity motor performance and motor recovery in stroke. In addition, another study reported that EA training showed significant improvement over simple, repetitive practices in upper-extremity motor recovery (Fugl–Meyer Assessment and Wolf Motor Function Test) post-stroke [16]. However, evidence for the effectiveness and therapeutic strategies of EA is still lacking. In other words, it is necessary to standardize therapeutic strategies through more diverse clinical studies [4]. Thus, the purpose of this study was to investigate the effect of upper-limb robot-assisted therapy based on visual error augmentation in virtual reality for motor recovery and kinematics after chronic hemiparetic stroke, providing a feasibility study.
2. Materials and Methods
2.1. Subjects
This study applied a single-group pre- and post-test study design to investigate the effect of upper-limb robot-assisted therapy based on visual error augmentation in virtual reality for motor recovery and kinematics in chronic hemiparetic stroke survivors. Twenty-seven stroke survivors volunteered to participate in this study. It was approved by the Korea National Rehabilitation Center Institutional Review Board (NRC-2018-02-010) and was conducted in accordance with the approved guidelines. All participants provided informed consent according to the Declaration of Helsinki prior to commencing the study. The inclusion criteria were as follows: (1) at least 6 months after stroke onset; (2) ability to follow the study instructions (≥24 points on the Korean version of the Mini-Mental State Examination); (3) absence of any musculoskeletal condition that could affect the ability to sit safely; and (4) presence of some recovery in the upper extremity (Fugl-Meyer Assessment score 15 to 50) [16]. Exclusion criteria were (1) shoulder subluxation or obvious joint pain of the upper extremity; (2) severe spasticity (modified Ashworth scale < 3); and (3) botulinum toxin injection to the paretic side of the upper extremity within 4 months.
2.2. Study Setting
Upper-limb robot-assisted therapy based on visual error augmentation in virtual reality was conducted with a national rehabilitation center end-effector-based rehabilitation arm at home (NREH), developed at the Korea National Rehabilitation Institute. The NREH is composed of a robot body, an end-effector-based robotic arm, and a visual display environment system in virtual reality (Figure 1A). The robot body is equipped with 4 casters that can be moved and fixed. In addition, the robot body can be adjusted in height (700–1100 mm) through an electric motor. The end-effector-based robotic arm has two degrees of freedom, a five-bar linkage, two torque motors, position encoders, and a handle that the subject can grasp. During upper-limb robot-assisted therapy based on visual error augmentation in virtual reality, the speed, distance, and trajectory of the handle movement were derived from two position encoders. In addition, two torque motors delivered a programmed assistive force to the subject that grasped the handle. An assistance force of 4 N was temporarily provided based on the subjects’ point-to-point movement performance to assist a lack of movement. The visual display environment was provided as virtual reality via a 32-inch monitor (LG Electronics, Seoul, South Korea, model 32QK500). The NREH was designed to adjust the height of the end-effector-based robotic arm and the angle of the visual display environment system, according to the subject’s sitting posture.
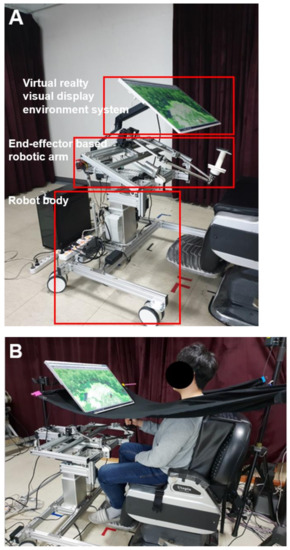
Figure 1. Configuration of NREH (A) and setting for upper-limb robot-assisted therapy based on visual error augmentation in virtual reality (B).
[…]
[ARTICLE] Home-based rehabilitation programs on postural balance, walking, and quality of life in patients with stroke – Full Text
Posted by Kostas Pantremenos in Gait Rehabilitation - Foot Drop, Tele/Home Rehabilitation on September 9, 2021
Abstract
Background
The most challenging aspect of rehabilitation is the high costs of in-patient rehabilitation programs and poor continuity of care while patients are transferred to home. In this regard, numerous home-based rehabilitation programs have been developed. The purpose of this study was to investigate the effects of home-based rehabilitative programs on postural balance, walking, and quality of life in individuals with chronic hemiparetic stroke.
Design
A CONSORT-compliant randomized controlled trial.
Methods
Seventeen community-dwelling people diagnosed with a first stroke participated in this study. They randomly divided the home-based rehabilitative program (HBP) group (n = 9) and control group (n = 8). The HBP group received coordination exercises at home and the control group received clinic-based exercises. This study measured postural balance, walking, and quality of life using four outcome measures: 10-meter walk test, figure of 8 walk test, four-square step test, and 36 item short-form survey.
Results
After analysis, it was found that the HBP improved postural balance, comfortable speed, and fast speed walking, and straight and curved walking for chronic stroke. Second, clinic-based rehabilitation services improved postural balance, comfortable speed, and fast speed walking abilities in patients with chronic stroke.
Conclusion
The results of this study suggest that the HBP group received positive benefits with regard to the postural balance and walking abilities of chronic hemiparetic stroke patients compared to the clinical setting exercise program.
1 Introduction
Stroke is a leading cause of permanent physical disabilities and, ultimately, death in adults, and permanent disabilities in stroke survivors face the need to improve their functional activities and quality of life for a long time, and also perhaps their life expectancy. They would experience daily struggles with physical, psychological, and financial problems in order to return to their previous social roles in life expectancy.[1,2] Another cause of the increase following the stroke was the limited resources in rehabilitation facilities. Medical costs are a burden to both patients and the healthcare policy services. Most stroke patients do not require long-term rehabilitation services because of their medical health insurance and the limited resources of the facilities.[2] Therefore, most stroke survivors leave the hospital and return to their home or community without requiring sufficient rehabilitative time for both patients and their families or caregivers to learn about their new life situations.[3]
Most studies try to facilitate strategies to decrease the high medical costs following stroke and to prepare stroke survivors for their successful return to ordinary life.[4–8] Home-based rehabilitative program (HBP) following stroke has recently been regarded as offering potential benefits compared to hospital rehabilitation.[4] There is growing rehabilitative service demand for home-based rehabilitative programs to improve disability and to integrate the community and social roles for stroke survivors.[6,7] The HBP changed from a focus on avoiding hospitalization to a focus on early discharge from care but with support from ongoing recovery by providing rehabilitation and other services in a community setting.
HBP for stroke patients has several advantages compared to care in rehabilitation centers. HBP would lower heavy medical costs and develop the efficacy of these services by using the patient’s own life circumstances. HBP has been found to induce active participation of families and caregivers and compensate for the lack of interactions between patients and therapists.[5,8] The HBP would positively affect the patient’s quality of life. Satisfaction with rehabilitation services is higher than that of hospital-based rehabilitation because HBP allows stroke patients to functionally practice their activities in a real environment, but within a standardized hospital environment.[9,10]
The HBP for stroke patients has three different aims: early supported discharge, replacing rehabilitation centers with home, and healthy promotion.[4] Olney et al[11] compared the outcomes achieved by two groups of people with chronic stroke, one with 10 weeks of supervised training, and one group given 1 week of supervised training to learn the program, followed by 9 weeks of unsupervised training carried out at home. Both groups made equally modest gains with regard to the indicators of motor impairment and cardiovascular risk and on physical and mental health.[11] Mayo et al subsequently designed a trial of HBP: a task-oriented exercise and walking program and a cycling regimen for a 1-year period. All patients were visited at home 13 times in 12 months and underwent regular telephone monitoring. Both groups had elements of repetitive training, but the cycling regimen was simpler, with more opportunities for continuous repetitive training. The cycle group would experience greater increases in walking ability, secondarily to developing better exercise habits and, consequently, greater gains in participation and health-related quality of life.[12] However, there is insufficient data to compare the effectiveness of HBP and clinical-based rehabilitation for chronic hemiparetic stroke patients.
This study investigated the effects of home exercise to prevent deterioration and promote physical activities as well as quality of life in patients with chronic hemiparetic stroke. The purpose of this study was to compare the effects of HBP and clinic-based rehabilitation on postural balance, walking, and quality of life in individuals with hemiparetic stroke. This study involved a coordination exercise program based on the rhythmic movements of the lower extremities as an HBP. The hypothesis of this study was that there would be a cost difference between HBP and clinical-based rehabilitation for chronic hemiparetic stroke patients.[…]
[Abstract] Multi-input Multi-output Fuzzy Logic Controller for Hybrid Exoskeleton and Functional Electrical Stimulation for Hand Movements Rehabilitation of Hemiparesis Patients
Posted by Kostas Pantremenos in Paretic Hand, Rehabilitation robotics on August 21, 2021
Abstract
Stroke is a clinical syndrome characterized by the sudden development of persistent neurological deficits focused secondary to vascular events. Stroke is the number one cause of disability in the world and the number two cause of death in the world. Two thirds of strokes occur in developing countries. The most common effect in post-stroke conditions is weakening of the upper limbs or hemiparesis, which can be seen in 77% of people who recover. Weakening of the limbs causes the patient to not be able to control hand movements to the maximum so that it affects the ability of individuals to carry out daily activities, one of which is the ability of individuals to hold objects. A hybrid system device of functional electrical stimulation (FES) and soft-exoskeleton is developed. Multiple-Input-Multiple-Output Fuzzy Logic Controller (MIMO-FLC) is used as the control system. There are two inputs, two outputs, and 28 fuzzy rules from the FLC. It was found that the use of MIMO-FLC can integrate exoskeleton and FES better than MISO-FLC in previous studies. The control system using MIMO-FLC is an appropriate and effective method for integrating two different FES and exoskeleton actuation systems.
[ARTICLE] Effects of lower extremity constraint-induced movement therapy on gait and balance of chronic hemiparetic patients after stroke: description of a study protocol for a randomized controlled clinical trial – Full Text
Posted by Kostas Pantremenos in Constraint induced movement therapy CIMT, Gait Rehabilitation - Foot Drop, REHABILITATION on July 24, 2021
Abstract
Background
Protocols involving intensive practice have shown positive outcomes. Constraint induced movement therapy (CIT) appears to be one of the best options for better outcomes in upper limb rehabilitation, but we still have little data about lower extremity constraint-induced movement therapy (LE-CIT) and its effects on gait and balance.
Objective
To evaluate the effects of an LE-CIT protocol on gait functionality and balance in chronic hemiparetic patients following a stroke.
Methods
The study adopts a randomized, controlled, single-blinded study design. Forty-two patients, who suffered a stroke, who were in the chronic phase of recovery (>6 months), with gait disability (no community gait), and who were able to walk at least 10 m with or without the advice or support of 1 person, will be randomly allocated to 2 groups: the LE-CIT group or the control group (intensive conventional therapy). People will be excluded if they have speech deficits that render them unable to understand and/or answer properly to evaluation scales and exercises selected for the protocol and/or if they have suffered any clinical event between the screening and the beginning of the protocol. Outcome will be assessed at baseline (T0), immediately after the intervention (T1), and after 6 months (T2). The outcome measures chosen for this trial are as follows: 6-min walk test (6minWT), 10-m walk test (10mWT), timed up and go (TUG), 3-D gait analysis (3DGA), Mini Balance Evaluation Systems Test (Mini-BESTest), and as a secondary measure, Lower Extremity Motor Activity Log will be evaluated (LE-MAL). The participants in both groups will receive 15 consecutive days of daily exercise. The participants in the LE-CIT group will be submitted to this protocol 2.5 h/day for 15 consecutive days. It will include (1) intensive supervised training, (2) use of shaping as strategy for motor training, and (3) application of a transfer package (plus 30 min). The control group will receive conventional physiotherapy for 2.5 h/day over 15 consecutive days (the same period as the CIT intervention). Repeated measures analyses will be made to compare differences and define clinically relevant changes between groups.
Results
Data collection is currently on-going and results are expected in 2021.
Discussion
LE-CIT seems to be a good protocol for inclusion into stroke survivors’ rehabilitation as it has all the components needed for positive results, as well as intensity and transference of gains to daily life activities.
Trial registration
www.ensaiosclinicos.gov.brRBR-467cv6. Registered on 10 October 2017. “Effects of Lower Extremities – Constraint Induced Therapy on gait and balance function in chronic hemipretic post-stroke patients”.
Background
Cardiovascular disease is the leading cause of death in the world, representing 31% of the total number of deaths in 2017 [1]. Stroke accounts for almost half of these deaths [1] which means that it is the second greatest cause of death around the world [2] and the third most common cause of disability [3]. Hemiplegia is often the most common sequel caused by stroke, compromising independence in mobility at home or in the community, which sometimes results in losing premorbid society roles and requiring care for a long period of time [4].
Studies have reported that 6 months after the injury, 30% of patients are still unable to walk without assistance [5,6,7], and 1 year after the event (with relatively good recuperation), half of these patients are still not able to complete the 6-min walk test, walking just 40% of the predicted distance [7]. Despite all rehabilitative efforts, 35% of patients with initial paralysis in lower limbs are still unable to recover a functional gait and 25% are not able to walk without external aid [8]. Thus, within physiotherapy services, the majority of interventions involve approaches to gait training [9].
Protocols involving intensive practice have shown positive outcomes. Constraint induced movement therapy (CIT) appears to be one of the best options for better outcomes in upper limb rehabilitation. Experimental studies in the 1960s using CIT demonstrated that monkeys that suffered sensory deafferentation of their forelimb and then acquired learned non-use were able to use that paw again after having their unimpaired limb constrained for a number of days [10].
A growing number of studies have since supported the efficacy of CIT in upper limb rehabilitation for patients with chronic hemiparesis caused by stroke, which has been recognized and recommended within the treatment sets for this population [11,12,13]. Moreover, it has been considered the most effective physiotherapy approach for getting better rehabilitation outcomes for paretic upper limbs [14, 15].
CIT has been defined as a “therapeutic package” consisting of different numbers of compounds of combined treatment, used in a systematic and integrated way to engage the patient in using their affected limb for many hours per day over 2–3 consecutive weeks [16]. One of the main advantages of CIT in relation to the various different approaches used in neurological rehabilitation is that it is focused on the behavioral aspects of the method (monitoring, self-efficacy, solving problems, and contractual intervention); this guarantees the active participation of patient during the entire protocol [16].
The current CIT protocol consists of 3 main elements with multiple components and sub-components: (1) repetitive and task-oriented training (diary training with supervision), (2) behavioral strategies (transference package), and (3) constraint of affected limb (for upper extremity protocol) and/or any method to constantly remind the participant to use their more affected limb [16,17,18].
Post-stroke patients submitted to the CIT protocol for upper extremities present notable changes in the central nervous system (CNS) with improvement in cortical activation and increase of brain areas, using transcranial magnetic stimulation [19,20,21] or functional magnetic resonance [22,23,24].
There are still few data about lower extremity constraint-induced movement therapy (LE-CIT). In 2013, a case series was published which had been conducted on multiple sclerosis patients with a 4-year follow-up. At the end of the protocol they observed that these patients showed a notable improvement in Lower Extremity Motor Activity Log (LE-MAL) [25].
Although the studies used modified CIT, its methodology was not fully applied. For instance, the intensity applied was lower than that defined by the protocol; the presence of physical constraint on the non-affected side is described (this was discarded as it can create a bigger asymmetry and more abnormal movement); structure of training built without citing shaping (approaching in small steps); adoption of only one exercise, or a simple combination of different therapeutic approaches such as Bobath, muscle strengthening, or climbing stairs [26]; absence of a transference package, or differences in its structure (making only one homework list with exercises instead of a new list every day with different functional tasks); absence of a behavioral contract; and control group not receiving the same intensity of training [27, 28].
Despite not adhering exactly to the recommended model of CIT, these studies observed positive results such as an improvement in motor function, mobility, dynamic balance, discharge weight symmetry, gait ability, gait speed, length and width of step, and force of foot ground contact [26, 28]. However, in view of the above information, the investigation of the effects of the original LE-CIT protocol on gait functionality and balance of chronic hemiparetic patients following a stroke was not completely clarified.
The following research question was established to examine the effects of LE-CIT vs intensive conventional therapy on gait functionality and balance, as well as the transference of these gains in therapy to the environment outside the clinical setting in chronic hemiparetic patients following a stroke: is LE-CIT more effective compared with intensive conventional therapy with regard to gait functionality and balance in people suffering from stroke?[…]
[BLOG] How to normalize gait – Dynamic AFO Foot Drop Brace after stroke
Posted by Kostas Pantremenos in Gait Rehabilitation - Foot Drop on June 18, 2021
Ankle foot orthoses (AFOs) are frequently prescribed to improve gait deviation and normalize walking patterns in patients with drop foot hemiplegia disorder. In healthy individuals, the functional lower limb shortening which is hip & knee flexion and ankle dorsiflexion mainly achieve toe clearance. By the way, reduction of toe clearance of the affected foot derived from ankle joint disability(foot drop) causes the abnormal movement of knee and hip hike to compensate for the ankle movement. It is a major cause of falls in patients with foot drop disorder.

Table of contents
- What are Dynamic AFOs?
- Who needs a Dynamic Ankle Foot Orthotic (DAFO)?
- Before buying an AFO, check if the drop foot brace…
- Neofect STEP Dynamic AFO
What are Dynamic AFOs?
Conventional AFOs are used to restrict ankle plantarflexion/dorsiflexion movement, thus maintaining the hemiparetic foot in a fixed position of dorsiflexion to facilitate swing. However, this ROM restriction in the ankle joint disrupts the rhythm of gait and increases energy consumption during walking. To alleviate this issue, hinge-applied AFOs were developed to allow some ankle flexibility during the loading response on the affected lower limb, thus slightly reducing the energy cost of hemiparetic gait. It is generally called Dynamic Ankle Foot Orthosis(DAFO). Dynamic Ankle Foot Orthosis(DAFO) generally refers to a custom-made Supra-Malleolar Orthosis fabricated from thin thermoplastic material. It fits the foot intimately and the flexible and thin thermoplastic use means that DAFO can provide circumferential control of the rear and forefoot to maintain a neutral alignment. In the original designs of DAFOs, a ‘neurological’ footplate was often incorporated that consisted of a pad at the peroneal & calf site with dorsiflexing the toes.
These days, the prefabricated DAFOs are common and those are usually applied with shoes to use in daily life especially in outdoor activities.

Who needs a Dynamic Ankle Foot Orthotic (DAFO)?
- Individuals who have had foot drop after stroke or other neurological deficits as well as nerve injuries resulting in abnormal gait.
- Those who need to regain the rhythm of gait and increase energy efficiency during walking.

Before buying an AFO, check if the orthosis…
- is tested by experts
- is easy to wear with shoes
- supports the ankle and keep dorsiflexed properly
- keeps the ankle and foot from inverting and dropping
- allows ankle mobility for a various movement like squat and lunge
- provides support only where it is needed, swapping unnecessary bulk for a slim fit and cosmetically-appealing look
- promotes balance improvement through correction of limb asymmetry and hip joint compensation
Neofect STEP Dynamic AFO

Neofect STEP is designed to support the ankle and keep the foot dorsiflexed by the Neofect’s expert physical therapist. The main purpose of AFO is to keep the ankle and foot from inverting and dropping at the initial contact and toe-off phase. Also, neutral foot positioning assist ensures a secure heel strike, while ankle joint stabilization corrects varus/valgus positioning in mid-stance. Dynamic dorsiflexion through the elastomer ankle joint ensures toe clearance during the swing phase. This can be caused by nerve injury, muscle or nerve disorders, brain or spinal cord disorders. With its flexible/supportive hinge component, an AFO helps individuals walk more naturally while lifting the foot and keeping the ankle in alignment.

Foot drop causes the toes to drag on the ground, creating the need for gait changes to compensate to clear the foot. It’s forcefully making you use those muscles that have atrophy, so you can see a difference when not wearing it. Also, it provides enough range of motion to still be able to squat, lunge and perform a higher level of balance. And the foot-plate supports approximately ⅔ of foot length. It retains flexibility and prevents paresthesia in the toes.
Neofect STEP provides a strong, lightweight solution to support people with a range of walking disorders caused by a variety of neurological and musculoskeletal disorders.
[Abstract] Constraint Induced Movement Therapy Increases Functionality and Quality of Life after Stroke
Posted by Kostas Pantremenos in Constraint induced movement therapy CIMT, Paretic Hand, REHABILITATION on April 16, 2021
Highlights
• CIMT reduced spasticity
• Motor function of the upper limb and functional range improved with CIMT
• CIMT changed the quality of life
• One hour daily for 3x a week and without additional restriction time was sufficient
• CIMT promoted better adherence compared to the original protocol.
Abstract
This blind randomized clinical trial evaluated the effect of CIMT on the functionality and quality of life (QOL) of chronic hemiparetics. Thirty volunteers were divided into two groups: Control (CG) and CIMT (CIMTG); evaluated before and after 12 and 24 intervention sessions. The scales used were: adapted Fugl-Meyer Motor Assessment (FMA), Modified Ashworth, Stroke Specific Quality Of Life (SS-QOL) and the Functional Reach Test (FRT). The scores for all FMA variables in the CIMTG increased until the 24th session, differing from the pre-treatment. In the CG, the scores increased for pain, coordination/ speed and sensitivity. In the FRT there was an increase in the scores in both groups; after the 12th and 24th sessions, the result of the CIMTG was superior to the CG. For the SS-QOL in the CIMTG, the general score and most of the variables increased, as well as in the CG. Muscle tone in CIMTG was lower compared to CG after 24 sessions. Both protocols used in the study were effective, the CIMT protocol showed benefits in recovering the functionality of the paretic upper limb, in the functional range and in reducing muscle tone, with a consequent improvement in quality of life.
[ARTICLE] Factors related to daily use of the paretic upper limb in patients with chronic hemiparetic stroke–A retrospective cross-sectional study – Full Text
Posted by Kostas Pantremenos in Paretic Hand on March 13, 2021
Abstract
Aims
The present study aimed to determine factors associated with the frequency of paralyzed upper extremity (UE) use in chronic stroke patients with severe UE functional deficiency.
Methods
We retrospectively reviewed the medical records of 138 consecutive patients, and 117 was analyzed (median age, 55 [range, 18–85] years; median stroke duration, 24.5 [range, 7–302] months) with chronic hemiparetic stroke who were admitted to our hospital for intensive upper extremity rehabilitation. The mean Fugl-Meyer Assessment (FMA) UE score was 28.6. All of them are independent in their activity of daily living (ADL) and without remarkable cognitive deficits. Amount-of-use score of Motor Activity Log-14 (MAL-AOU) was applied as the index of daily use of affected UE. The following parameters were examined as the explanatory variables: demographics, proximal and distal sub-scores of FMA UE, Modified Ashworth Scale (MAS), and sensory function scores in the Stroke Impairment Assessment Set (SIAS).
Results
The median MAL-AOU score was 0.57 [range, 0.28–0.80]. Ordinal regression analysis revealed that FMA proximal, FMA distal, and SIAS sensory function (touch) were associated with AOU score of MAL-14 (Pseudo R-square = 0.460).
Conclusion
Not only motor but also sensory function, especially tactile sensation, play a crucial role in the daily use of affected UE in chronic stroke patients with severe UE hemiparesis.
Introduction
In chronic stroke patients, the amount of affected upper extremity (UE) use is important not only to maintain functional ability but also prevent “learned nonuse” and subsequent functional deterioration [1]. Although a growing number of reports demonstrate the efficacy of specific intensive approaches on certain aspects of disability, particularly regarding UE function, it is noteworthy that the benefit is in general limited for the patients with chronic stroke [2]. On the other hand, approaches to proceed a self-management or home-based rehabilitation has a certain effect not only to maintain chronic health condition, but also to improve body function [3,4]. It is suggested that tailored counselling or with tailored supervised training as well as nurses’ intervention improves participation and arm function [5,6].
To design a more personalized home-based rehabilitation program, it is imperative to know the factors affecting paretic UE disuse in activities of daily living (ADL) in the chronic stage. Such factors may be the treatment target of telehealth intervention [7]. Researchers have reported a predictability of daily use or activity limitation in the chronic phase using motor functional profile in the sub-acute phase [8,9], but it should be noted these studies reported indirect relationship in the different phases of stroke. Just two other studies have examined the contributions of motor function and proprioception individually in single factorial analyses [10,11]. Thus, no study has so far attempted to reveal the direct real-time causal relationship between UE use and clinically important impairment profiles, including motor function, spasticity, tactile sensation, and proprioception, with multifactorial analysis [12]. Subsequently, a responsive intervention to improve the participation of upper extremity targeting specific aspect of sequelae remains difficult.
On these grounds, the present retrospective cross-sectional study aimed to determine which clinical factors influence the paretic UE participation in chronic hemiparetic stroke patients, particularly those with severe motor and mild-to-moderate sensory disturbances. […]
[ARTICLE] Novel design for a dynamic ankle foot orthosis with motion feedback used for training in patients with hemiplegic gait: a pilot study – Full Text
Posted by Kostas Pantremenos in Gait Rehabilitation - Foot Drop on January 19, 2021
Abstract
Background
We designed a novel ankle foot orthosis (AFO), namely, ideal training AFO (IT-AFO), with motion feedback on the hemiparetic lower limb to improve ambulation in individuals with stroke-related hemiplegia. We, therefore sought to compare the kinematic parameters of gait between IT-AFO with and without dynamic control and conventional anterior-type AFO or no AFO.
Methods
Gait parameters were measured using the RehaWatch® system in seven individuals with hemiplegia (mean 51.14 years). The parameters were compared across four conditions: no AFO, conventional anterior AFO, IT-AFO without dynamic control, and IT-AFO with dynamic control, with three trials of a 10-m walk test for each.
Results
The dorsiflexion angle increased during the swing phase when the IT-AFO was worn, and it was larger with dynamic control. These data can confirm drop foot improvement; however, the difference between the parameters with- and without-AFO control conditions was not significant in the swing phase. The IT-AFO with or without dynamic control enhanced the loading response to a greater extent between the hemiparetic and unaffected lower limbs than conventional AFO or no AFO. The duration of the stance phase on the hemiparetic lower limb was also longer when using IT-AFO with and without dynamic control than that when using conventional AFO, which improved asymmetry. User comfort and satisfaction was greater with IT-AFO than with the other conditions.
Conclusions
The IT-AFO with dynamic control improved gait pattern and weight shifting to the hemiparetic lower limb, reducing gait asymmetry. The difference with and without dynamic control of IT-AFO is not statistically significant, and it is limited by sample size. However, this study shows the potential of IT-AFO in applying positive motion feedback with gait training.
Trial registration
Taipei Medical University-Joint Institutional Review Board. N201510010. Registered 12 February 2015. http://ohr.tmu.edu.tw/main.php.
Background
An ankle foot orthosis (AFO) is a commonly used device to improve gait in patients with stroke-related hemiplegia [1]. An AFO provides physical support to the ankle joint and foot [2], with the aim of improving weight-bearing on the affected lower limb. It is estimated that over 4 million people in the United States use an AFO for gait-related impairments [3]. The American Board for Certification in Orthotics, Prosthetics and Pedorthics, Inc. (ABC) reported that in 2016, 74.2% of orthotists’ time was spent in fabricating lower limb orthoses, with AFOs accounting for 36% of these devices. Similarly, in 2014, the Social and Family Affairs Administration in Taipei reported that the highest proportion of subsidies were for AFOs [4].
Stroke is the most common indication for AFO prescription. A stroke is defined as the death of brain cells caused by cerebral ischemia, which results in a wide range of motor impairments, including gait impairment [5]. The prevalence of stroke is approximately 19.3 per 1000 people aged over 35 years in Taiwan [6]. In the United Sates, it is estimated that 795,000 people sustain a new or recurrent stroke every year [7]. Gait function is often affected in stroke survivors [8,9,10], with AFOs recommended to improve the position of the foot and ankle during the gait cycle [11]. A retrospective analysis concluded that the prevalence rate of AFO use after a stroke was 30.7% in Japan in 2015, with a better Functional Independence Measure score at discharge among patients who were prescribed an AFO than that in patients who did not use an AFO for gait retraining [12].
Conventional AFOs are used to restrict ankle plantarflexion, thus maintaining the hemiparetic foot in a position of dorsiflexion to facilitate swing [13]. However, this restriction in ankle movement disrupts the rhythm of gait and increases energy consumption during walking [14,15,16]. To alleviate this issue, hinge AFOs were developed to allow some dorsiflexion during the loading response on the affected lower limb, thus slightly reducing the energy cost of hemiparetic gait [17]. Elastic materials (such as carbon fibers) have been included in some AFO designs to provide an assistive function to further reduce energy expenditure [18, 19]. Mechanical features (such as dampers and springs) as well as electronic components (such as magnetorheological braking systems, force and position sensors, accelerometers, and microprocessors) have been included in the hinge to try and improve control over ankle motion [19, 20]. However, to the best of our knowledge, an AFO has not been developed with the specific aim of providing motion feedback for gait training.
Typically, physical therapists use hands-on activities as feedback to facilitate normal movement patterns in conventional gait training [21]. Recently several devices provide “reminding” external feedback for improving gait performance, such as stance-feedback to increase the stance time on the affected side or swing-feedback to decrease the swing time on the affected side [22, 23]. Therefore, we designed an AFO with novel motion feedback mechanism, which is performed by recognition execution of motor learning.
In this study, we describe a novel type of AFO, the ideal training AFO (IT-AFO), which we developed at Taipei Medical University and customized in a patient-specific manner using 3-dimensional (3D) printing for fabrication [24, 25]; it optimizes the alignment of the hinge with the axis of motion of the ankle in the sagittal plane. The IT-AFO includes a dynamic component designed specifically to provide motion feedback during walking. This mechanism design is based on changes in the ankle angle during the gait cycle [26]. There are two dynamic components on both sides of one IT-AFO, and each dynamic component contains a one-way damper (1 Ns/m) and a spring (0.625 kgf), shown in Fig. 1a. Springs are only used to restore components while dampers provide the main plantarflexion resistance. However, springs provide very little plantarflexion resistance during the swing phase (Fig. 1b). Springs can retract the straps when in the stance phase with sufficient weight shifting to the affected side resulting in component restoration (Fig. 1c). Insufficient weight shifting to the affected side decreases the ankle dorsiflexion angle, because straps are still tight when in the stance phase, thus impeding component restoration (Fig. 1d) (straps are still tight because of insufficient ankle dorsiflexion). Users will feel more assisted force on the swing phase after every step with sufficient weight shifting to the affected side on the stance phase. Therefore, IT-AFO has potential for enhancing motor control recognition schema with gait training.
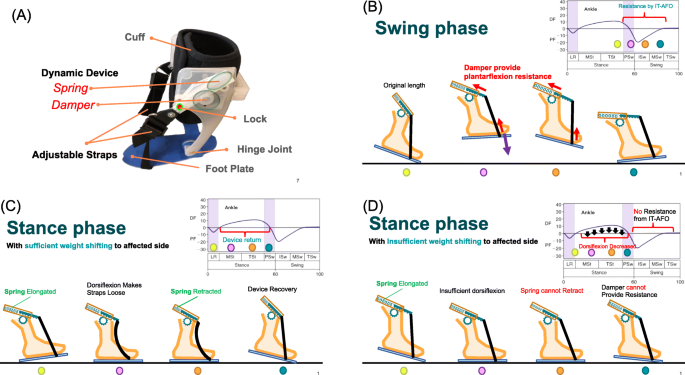
This study aimed to compare the gait kinematics in individuals with stroke-related hemiplegia using IT-AFO with dynamic control, IT-AFO without dynamic control, conventional AFO, and no AFO. […]
[Abstract] Muscle and tendon properties of the spastic lower leg after stroke defined by ultrasonography: a systematic review
Posted by Kostas Pantremenos in Gait Rehabilitation - Foot Drop, Spasticity on December 24, 2020
Abstract
Introduction: Peripheral muscle and tendon changes after stroke can influence the functional outcome of patients. The aim of this systematic review was to summarize the evidence of ultrasonographic changes in morphological muscle and tendon properties of the spastic hemiparetic lower leg in patients with first ever stroke.
Evidence acquisition: A systematic search was conducted through PubMed, Embase, Scopus, Cinahl, Cochrane Library, and manual searches from inception until 1st of May 2020. Observational case control or cohort studies were included. Risk of bias was evaluated by using the Newcastle-Ottawa Quality Assessment Scale. Outcome parameters of interest included muscle thickness, muscle and tendon length, fascicle length, pennation angle and echo-intensity.
Evidence synthesis: Nine studies investigated outcome parameters beyond one-month after stroke. We are unable to make a comprehensive statement. Nevertheless, there are some arguments for reduced muscle thickness and reduced fascicle length of the hemiplegic, spastic leg.
Conclusions: Despite the fact that objective assessment by ultrasonography holds promise for diagnosis and follow-up of spastic hemiparesis after stroke, more evidence is needed to determine how changes in morphological muscle and tendon properties are related to muscle weakness, severity of spasticity and compensation strategies such as disuse or overuse in longitudinal studies starting early after stroke.
