Archive for category Gait Rehabilitation – Foot Drop
[ARTICLE] Noninvasive spinal stimulation improves walking in chronic stroke survivors: a proof-of-concept case series – Full Text
Posted by Kostas Pantremenos in Gait Rehabilitation - Foot Drop on April 8, 2024
Abstract
Background
After stroke, restoring safe, independent, and efficient walking is a top rehabilitation priority. However, in nearly 70% of stroke survivors asymmetrical walking patterns and reduced walking speed persist. This case series study aims to investigate the effectiveness of transcutaneous spinal cord stimulation (tSCS) in enhancing walking ability of persons with chronic stroke.
Methods
Eight participants with hemiparesis after a single, chronic stroke were enrolled. Each participant was assigned to either the Stim group (N = 4, gait training + tSCS) or Control group (N = 4, gait training alone). Each participant in the Stim group was matched to a participant in the Control group based on age, time since stroke, and self-selected gait speed. For the Stim group, tSCS was delivered during gait training via electrodes placed on the skin between the spinous processes of C5–C6, T11–T12, and L1–L2. Both groups received 24 sessions of gait training over 8 weeks with a physical therapist providing verbal cueing for improved gait symmetry. Gait speed (measured from 10 m walk test), endurance (measured from 6 min walk test), spatiotemporal gait symmetries (step length and swing time), as well as the neurophysiological outcomes (muscle synergy, resting motor thresholds via spinal motor evoked responses) were collected without tSCS at baseline, completion, and 3 month follow-up.
Results
All four Stim participants sustained spatiotemporal symmetry improvements at the 3 month follow-up (step length: 17.7%, swing time: 10.1%) compared to the Control group (step length: 1.1%, swing time 3.6%). Additionally, 3 of 4 Stim participants showed increased number of muscle synergies and/or lowered resting motor thresholds compared to the Control group.
Conclusions
This study provides promising preliminary evidence that using tSCS as a therapeutic catalyst to gait training may increase the efficacy of gait rehabilitation in individuals with chronic stroke.
Trial registration NCT03714282 (clinicaltrials.gov), registration date: 2018-10-18.
Background
Stroke is the leading cause of adult-onset disability [1]. Despite many advances in gait research in the last decade, about 35% of stroke survivors fail to regain independence in performing activities of daily living due to the impaired function of their affected leg, and about 70% have gait deficits, including reduced walking speeds, asymmetrical walking patterns, and motor coordination issues [2,3,4].
Walking deficits after stroke mostly derive from a disruption of the corticospinal pathways that play an important role in transmitting sensory–motor commands [5, 6]. To address this, most interventions using non-invasive electrical pulses focus on stimulation of the motor cortex to activate dormant or new pathways [2, 7, 8]. However, while supra-spinal regions can facilitate fine locomotor control, spinal networks ultimately generate the basic locomotor pattern [9, 10]. More interestingly, a recent study using functional MRI showed increased blood-oxygen-level dependent activities in motor cortex following transcutaneous spinal cord stimulation (tSCS) in individuals with stroke [11]. Therefore, we hypothesized that tSCS would facilitate an improvement of gait after stroke. Our previous work, in collaboration with additional researchers, established anatomical and physiological changes in the spinal cord after stroke [12, 13], offering a theoretical basis for testing our hypothesis of targeting the spinal circuits for post-stroke recovery.
Recently, Moshonkina et al. reported functional improvements in post-stroke individuals after 2 weeks of tSCS with standard physical therapy, achieving the minimum clinical important differences (MCID) in the 6 min walk test and comfortable walking speed [14]. The same investigators reported immediate improvements in walking kinematics after a single tSCS session [15, 16]. Notably, however, none of the studies mentioned above investigated the effects of more than 4 weeks of training nor tried to explore the potential neurophysiological differences accompanied with gait outcomes. Consequently, it remains unclear whether tSCS can exert a lasting impact on restoration of function following a stroke.
We investigated whether tSCS combined with symmetry-focused gait training has a sustained effect on gait recovery after chronic stroke. We hypothesized that longer-term gait training (24 sessions) with tSCS would lead to greater sustained improvements in walking function compared to control treatment focused solely on gait training. Specifically, we focused on gait symmetry since such improvements can have lasting effects on balance and overall mobility of stroke survivors [6]. We also expected that gait improvements would be associated with physiological changes in muscle coordination measured from electromyography (EMG) of the paretic side, and spinal excitability determined by the spinal motor evoked responses (sMERs). […]
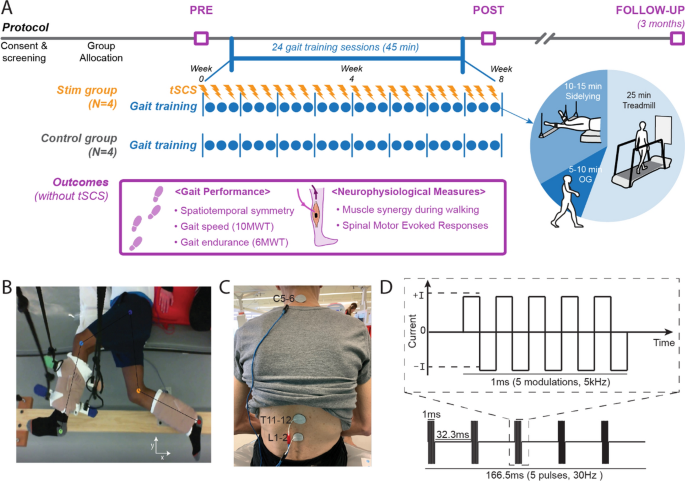
Study protocol and stimulation setup. A Overall experimental protocol. B Top–down view of position of the legs extended beyond the edge of the table and supported with vertically cables during the side-lying training of a participant (Stim 2). C tSCS delivered using surface electrodes on the skin between the C5–6, T11–12, and L1–2 spinous processes (cathode) and a surface electrode on each anterior crest (anode, not shown). D Schematic representation of biphasic pulse sequence used for tSCS. tSCS transcutaneous spinal cord stimulation, OG overground walking, 10MWT 10-m walk test, 6MWT 6-min walk test
[Preprint] Feasibility of Simultaneous Transcranial Direct Current Stimulation During Gait Training in Chronic Stroke Patients: A Randomized Double-blind Pilot Clinical Trial
Posted by Kostas Pantremenos in Gait Rehabilitation - Foot Drop, tDCS/rTMS on April 2, 2024
Abstract
Background
Transcranial direct current stimulation (tDCS) is a therapeutic tool for improving post-stroke gait
disturbances, with ongoing research focusing on specific protocols for its application. We evaluated the
feasibility of a rehabilitation protocol that combines tDCS with conventional gait training.
Methods
This was a randomized, double-blind, single-center pilot clinical trial. Patients with unilateral hemiplegia
due to ischemic stroke were randomly assigned to either the tDCS with gait training group or the sham
stimulation group. The anodal tDCS electrode was placed on the tibialis anterior area of the precentral
gyrus while gait training proceeded. Interventions were administered 3 times weekly for 4 weeks.
Outcome assessments, using the 10-meter walk test, Timed Up and Go test, Berg Balance Scale,
Functional Ambulatory Scale, Modified Barthel Index, and EQ-5D-3L, were conducted before and after the
intervention and again at the 8-week mark following its completion. Repeated-measures ANOVA was used for comparisons between and within groups.
Results
Twenty-six patients were assessed for eligibility, and 20 were enrolled and randomized. No significant
differences were observed between the tDCS with gait training group and the sham stimulation group in
gait speed after the intervention. However, the tDCS with gait training group showed significant
improvement in balance performance in both within-group and between-group comparisons. In the
subgroup analysis of patients with elicited motor-evoked potentials, comfortable pace gait speed
improved in the tDCS with gait training group. No serious adverse events occurred throughout the study.
Conclusions
Simultaneous tDCS during gait training is a feasible rehabilitation protocol for chronic stroke patients
with gait disturbances.
Introduction
Impairment of independent gait is one of the most disabling consequences after a stroke [1]. Gait
abnormality in stroke patients arises from a complex interplay of factors, including lower limb motor
weakness and decreased balance. Some individuals may not be entirely incapable of walking, but they
may still require gait aids or assistance from caregivers. Gait disturbances pose a greater risk of further
injury due to falls. It is well known that fall-associated fractures result in significant socioeconomic costs
[2]. Additionally, gait disturbances lead to limitations in social activity, thereby reducing the quality of life
of stroke patients. Therefore, improving gait performance in stroke patients has long been a desire shared
by patients and physicians.
Transcranial direct current stimulation (tDCS) is a non-invasive brain stimulation technique that aims to
modulate the human brain by delivering low-intensity electrical current through the scalp. The mechanism of tDCS is explained by 2 principles: (1) the enhancement of cortical activity through a polarity shift in the resting membrane potential and (2) the upregulation of neural plasticity through long-term potentiation [3]. Applying anodal tDCS to patients with subacute stroke has been associated with beneficial effects on motor function [4]. However, inconsistent results have emerged across studies, with some failing to observe significant improvements in patients who underwent tDCS compared to sham stimulation [5].
Additionally, there has been substantial variability in factors, such as stimulation area, intensity, duration,
and the number of sessions among different tDCS protocols, highlighting the need for further research to
establish an optimal tDCS protocol for maximizing the effect of conventional gait training methods.
Studies focusing on stimulation timing suggest that combining tDCS with gait training simultaneously
shows more promising results in improving gait performance compared to protocols that administer gait
training and tDCS separately [6].
This study aimed to evaluate the feasibility of a rehabilitation protocol that combines simultaneous tDCS
with conventional gait training and to investigate its impact on gait performance in chronic stroke
patients.[…]
[WEB] Discover the C-Brace: The Revolution in Orthotics
Posted by Kostas Pantremenos in Gait Rehabilitation - Foot Drop, REHABILITATION, Video on January 27, 2024
The C-Brace stands as the pioneering mechatronic Stance and Swing Phase Control Orthosis (SSCO®) system in the world. It skillfully combines hydraulic control of both stance and swing phases with advanced microprocessor sensor technology. Unlike traditional paralysis orthoses, which only offer knee joint locking and releasing, the C-Brace delivers continuous support throughout the entire gait cycle, adjusting in real-time to various daily activities.
Key Highlights of the C-Brace:
- Unparalleled Innovation: As the only intuitive Knee-Ankle-Foot Orthosis (KAFO) of its kind, the C-Brace stands out from alternatives that require more effort and manual intervention for daily use.
- Adaptive and Responsive: The C-Brace uniquely learns and adapts to your patients’ movements, offering unmatched stability and confidence in their everyday activities.
- Hands-Free Mobility: With its groundbreaking technology, the C-Brace allows users complete hands-free mobility across various actions – be it walking, sitting, standing, or navigating stairs and ramps.
Empirical Evidence of Efficacy (2023 Study):
- Enhanced Balance: Users experienced a notable 20% improvement in balance with the C-Brace, compared to conventional KAFOs.
- Substantial Fall Reduction: A significant 73% decrease in falls was observed, markedly reducing users’ fear of falling.
- Quality of Life Improvements: Participants reported marked enhancements in 5 out of 9 quality of life areas, including a 50% increase in Physical Functioning, as per the global quality of life assessment.
- Reduced Dependence on Walking Aids: The study noted a considerable decrease in the need for walking aids during everyday activities.
For more comprehensive clinical data, download the C-Brace Evidence Essentials here.
Tailored for Individual Needs:
Each C-Brace is meticulously crafted to fit the unique anatomical requirements and specific conditions of the patient. This personalized approach ensures an optimal fit and function, involving several steps for evaluation and fitting for each potential user.
Identifying Suitable Candidates:
Our comprehensive list of indications assists in pinpointing the ideal candidates for the C-Brace. If you have a patient who might benefit from this innovative orthosis, please use the link below to request more information and arrange an in-person demo.
For Medical Professionals:
If you’re a healthcare professional seeking detailed information, please complete the form below and a dedicated Ottobock representative will contact you to provide personalized assistance.
Who is the C-Brace for?
Cognitive requirements
- The patient must be capable of ensuring the proper handling, care, and use of the orthosis (e.g. charging the battery, operating the user app, etc.)
Functional deficit
- Neuromuscular or orthopedic instability of the knee joint in the sagittal plane diagnosis (by the physician)
Has one or more of the following conditions:
- Quadricep weakness
- Charcot Marie Tooth Syndrome (CMT)
- Multiple Sclerosis
- Polio
- Spinal Cord Injury
- Hip/Knee/Ankle Injury
- Guillain-Barre Syndrome
- Stroke victim
- Deep vein thrombosis (DVT)
- Paraplegic
- Chronic inflammatory demyelinating polyneuropathy (CIDP)
- Transverse/Partial Myelitis
- Neuro Lyme Disease
- Fibromyalgia
- Osteogenesis Imperfecta
- Ehlers Danlos Syndrome
Disclaimer: Not all people with one or more of these conditions will be a candidate for C-Brace but should still be considered.
Indications:
The C-Brace may be considered for patients with all neurologic conditions resulting in paresis or paralysis of the knee extensors, or orthopedic conditions in which the quadriceps fail to keep the knee extended during stance phase who do not present any of the contraindications. The leading indications are incomplete paraplegia with very minor or no spasticity, as well as post-polio syndrome, the condition following poliomyelitis.
Other factors to consider:
- The patient must be able to fully stabilize their torso.
- The muscle strength of their hip extensors and flexors must permit controlled swing-through of the affected leg.
- Compensatory hip movement is permissible.
- The patient must fulfill the physical and mental requirements for perceiving optical/acoustic signals and/or mechanical vibrations.
Contraindications:
- Inability to advance the limb through compensatory motion or grade 3 hip flexor
- Insufficient trunk stability
- Moderate to severe spasticity
- A flexion contraction of more than 10° in the knee and/or hip joint
- Genu varus/valgus of more than 10° that cannot be corrected
- Body weight > 275 lbs.
- Leg length discrepancy > 6 in.
Videos
C-Brace Playlist – Therapy Exercises
Life with the C-Brace
Hannah
Melvin
Wolfgang
C-Brace Gait Comparison Videos
David
Denise
Heike
Melvin
Rebecca
[Abstract + References] Effects of robotic-assisted gait training on physical capacity, and quality of life among chronic stroke patients: A randomized controlled study
Posted by Kostas Pantremenos in Gait Rehabilitation - Foot Drop, Rehabilitation robotics on January 24, 2024
Abstract
Background
Even though robotic therapy is becoming more commonly used in research protocols for lower limb stroke rehabilitation, there still is a significant gap between research evidence and its use in clinical practice. Therefore, the present study was designed assuming that the wearable mobile gait device training for chronic stroke patients might have different effects on functional independence when compared to training with a stationary gait device. The present study aims to examine the effects of gait training with ExoAthlet exoskeleton and Lokomat Free-D on functional independence, functional capacity, and quality of life in chronic stroke patients.
Methods
The present study included 32 chronic stroke patients. Participants were randomly divided into two groups. Functional independence of patients was evaluated by using Functional Independence Measure (FIM), physical function was assessed by using the 30-second chair stand test (30-CST), functional capacity was measured by using the 6-Minute Walk Test (6MWT), and quality of life was assessed by using Short Form 36 (SF36). All participants underwent a conventional physiotherapy program for eight weeks, three sessions per week, and each session lasted 60 min. After the physiotherapy program, one group received gait training by using ExoAthlet exoskeleton (ExoAtlet 1 model/2019, Russia), while the other group received training by using Lokomat Free-D (Hocoma, Lokomat Pro Free-D model/2015, Switzerland). Participants were assessed at baseline and post-intervention.
Results
Results achieved in this study revealed that there was a statistically significant difference between FIM, 30-CST, 6MWT, and SF36 scores before and after the treatment in both groups (p < 0.05).There was no difference in FIM, 30-CST, and 6MWT results between Exoskeleton ExoAthlet and Lokomat Free-D groups (p > 0.05). However, there was a statistically significant difference between Exoskeleton ExoAthlet and Lokomat Free-D groups in terms of SF-36 sub-parameters “vitality”, “mental health”, “bodily pain”, and “general health perception” (p < 0.05).
Conclusions
This study demonstrated that the use of ExoAthlet exoskeleton and Lokomat Free-D in addition to conventional physiotherapy, was effective in improving functional independence, physical function, functional capacity, and quality of life among chronic stroke patients. Incorporation of robotic gait aids into rehabilitation for chronic stroke patients might offer significant advantages.
Introduction
Stroke is defined as a condition that develops due to a disturbance in brain functions, either in a specific location or in the whole brain, rapidly manifests symptoms, and these symptoms persist for one day or longer, or result in death [1]. Motor functions are affected in 65 % of chronic stroke patients, and the majority of these patients experience a reduced level of functional independence [2]. It is believed that the most prominent challenges faced by chronic stroke patients are the distance walked in 6 min and the decrease in functional capacity [3]. In addition, cohort studies also reported that 22 % of chronic stroke patients did not regain any walking function [4].
A significant portion of chronic stroke patients suffer not only from physical disability but also from cognitive and emotional disorders [5]. General predictors of the poor quality of life after a stroke were reported to include medical comorbidities, loss of physical function, social role difficulties, emotional involvement, and depression [6], [7], [8].
In recent years, robotic technology has exhibited notable advancement thanks to faster and more powerful computers, innovative computational methodologies, and a broader range of electromechanical components. This technological advancement also made robotics suitable for rehabilitation interventions, and robotic rehabilitation is a promising method for treating patients with motor disorders. Its significance lies in its the potential to increase and carefully control the dosage of therapy [9], [10]. However, robotic rehabilitation does not solely focus on augmenting the quantity and intensity of treatment.
Robotic systems not only generate simple and repetitive stereotypical movement patterns but can also be utilized in order to provide patients with more intricate and controlled multisensory stimuli [11]. It can be seen that the effect of rehabilitation technology on functional outcomes can be optimized by affording the nervous system greater opportunities to experience genuine activity-related sensorimotor input [12]. Nevertheless, there are ongoing studies examining the therapeutic effectiveness of robotic rehabilitation. In clinical rehabilitation practices addressing chronic stroke patients, robotic technologies are employed for gait training, providing opportunities to move freely on a stationary or mobile basis. Robotic devices are utilized as assistive, rehabilitative, and augmentative instruments in lower extremity rehabilitation for neurological conditions [13]. Considering the lower extremity rehabilitation, the Lokomat, utilized as a stationary device, was demonstrated to be effective in improving walking quality, speed, and balance in conditions such as Multiple Sclerosis, Cerebral Palsy, Parkinson’s disease, Brown-Sequard syndrome, and vascular dementia [14]. Moreover, Lokomat was also reported to be an effective approach used in the rehabilitation of chronic stroke patients. In the literature, a retrospective case-control study examined the efficacy of Lokomat Free-D on functional independence, functional capacity, and balance in chronic stroke patients [15]. It was also reported that Lokomat Free-D is effective in chronic stroke individuals, not only affecting motor functions such as walking, balance, muscle strength, walking ability, and speed but also cognitive and emotional status. [14]. However, to the best of our knowledge, there is no study examining the effectiveness of Lokomat on the quality of life in chronic stroke individuals could be found. Another method utilized for lower extremity rehabilitation is the use of mobile gait devices. Devices such as MIRAD, XoR, and ExoAthlet exoskeleton are some of them [13]. The ExoAthlet exoskeleton was found to increase gait speed and stability, as well as reducing body sway, in conditions such as Multiple Sclerosis and spinal cord injuries [16], [17]. In a randomized controlled study comparing ExoAthlet exoskeleton and traditional physiotherapy in chronic stroke patients, the ExoAthlet exoskeleton group exhibited significant improvements when compared to the traditional physiotherapy group. The ExoAthlet exoskeleton group had a decrease in hemiparesis severity, an increase in paretic limb muscle strength, improvement in balance, and notable enhancements in the walking process and speed [18]. However, there is no study available that examines the effectiveness of the ExoAthlet exoskeleton on quality of life in chronic stroke patients. In both literature and clinical practice, there are gaps concerning the effectiveness of Lokomat Free-D and ExoAthlet exoskeleton on different parameters. There is no study available that has comparing these two different methods among chronic stroke patients. Finally, this study aims to examine the effects of gait training with the ExoAthlet exoskeleton and Lokomat Free-D on functional independence, functional capacity, and quality of life in chronic stroke patients, as well as to investigate whether there are any different effects.
Section snippets
Study design and ethics
Study design: Randomized Controlled Study.
Ethics: The study protocol was approved by from the institutional ethics board of Üsküdar University’s Non-Interventional Ethics Committee (Approval no = 61351342/February 2021-66/26.02.2021). The present study was carried out in accordance with the principles outlined in the Declaration of Helsinki. Participants, who voluntarily agreed to participate in this study, signed a written consent form. The paper is registered with ClinicalTrials.gov, and the
Results
Initially, 40 chronic stroke patients were involved in the study, and 8 individuals did not meet the inclusion criteria. Thus, the study was completed with a total of 32 individuals. The flow chart of the study is presented in Fig. 3.
The sociodemographic characteristics of the chronic stroke patients included in the study are shown in Table 1.
The results of this study showed that there was a statistically significant difference between functional independence, functional capacity, and quality
Discussion
Significant advancements have been achieved in robotic technology, especially in the last ten years, and the use of robotic technology in healthcare has increased. The use of robotic technology increased in post-stroke rehabilitation due to its advantages such as performing movements very similar to normal activity, providing continuous stimulation of the central nervous system, and creating treatment options with appropriate intensity and dosage for the patient during the rehabilitation
Limitations and future directions
The study has some limitations. The long-term effects of robotic-assisted gait training were not evaluated in this study. Additionally, different psychometric properties that would affect the quality of life were ignored. Future studies taking these parameters into consideration may provide different perspectives on the interpretation of results.
Conclusion
In conclusion, the results achieved in this study showed that gait training with Exoskeleton ExoAthlete and gait training with Lokomat Free-D for eight weeks administered in addition to conventional physiotherapy have positive effects on functional independence, functional capacity, and quality of life parameters in post-stroke patients. Training with Exoskeleton ExoAthlet had a more positive effect on the quality-of-life sub-parameters, such as vitality, mental health, bodily pain, and general
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References (45)
- S. Wist et al.Muscle strengthening for hemiparesis after stroke: A meta-analysisAnn Phys Rehabil Med(2016)
- H.S. Jørgensen et al.Recovery of walking function in stroke patients: the Copenhagen Stroke StudyArch Phys Med Rehabil(1995)
- R.J. NudoFunctional and structural plasticity in motor cortex: implications for stroke recoveryPhysical Medicine and Rehabilitation Clinics(2003)
- I. Schwartz et al.The effectiveness of locomotor therapy using robotic-assisted gait training in subacute stroke patients: a randomized controlled trialPMR(2009)
- GBD. Stroke collaborators. global, regional, and national burden of stroke, 1990-2016: a systematic analysis for the…
- Clara Selves, Gaëtan Stoquart, Thierry Lejeune. Gait rehabilitation after stroke: review of the evidence of predictors,…
- J.P.L. Slenders et al.Early cognitive and emotional outcome after stroke is independent of discharge destinationJ Neurol(2020)
- G.O. Vincent-Onabajo et al.Consistent determinants of health-related quality of life in the first 12 months after stroke: a prospective study in NigeriaTop Stroke Rehabil(2015)
- A.C. Jonsson et al.Determinants of quality of life in stroke survivors andtheir informal caregiversStroke(2005)
- Samsa GP, Matchar DB. How strong is the relationshipbetween functional status and quality of life among personswith…
- A. Esquenazi et al.Robotic-assisted gait training and restorationAm J Phys Med Rehabil(2012)
- L. PignoloRobotics in neuro-rehabilitationJ Rehabil Med(2009)
- A.B. KeelingUnderstanding Stroke Rehabilitation Progression in a Robotic Rehabilitation TrialCumming School of Medicine(2020)
- H. Lee et al.Lower limb exoskeleton systems—overviewWearable Robotics(2020)
- R.S. Calabrò et al.Robotic gait rehabilitation and substitution devices in neurological disorders: where are we now?Neurol Sci(2016)
- A. Manuli et al.Calabrò RS.J Is intensive gait training feasible and effective at old age? A retrospective case-control study on the use of Lokomat Free-D in patients with chronic strokeClin Neurosci(2021 Oct)
- S.V. Kotov et al.The efficacy of the exoskeleton ExoAtlet to restore walking in patients with multiple sclerosis. Zhurnal nevrologii i psikhiatrii imeni SSKorsakova(2017)
- E.Y. Shapkova et al.Exoskeleton walk training in paralyzed individuals benefits from transcutaneous lumbar cord tonic electrical stimulationFront Neurosci(2020)
- S.V. Kotov et al.Efficiency of leg exoskeleton use in rehabilitation of cerebral stroke patientsSerbian journal of experimental and clinical research(2021)
- R. Mustafaoglu et al.Does robot-assisted gait training improve mobility, activities of daily living and quality of life in stroke? A single-blinded, randomized controlled trialActa Neurol Belg(2020)
- A.A. Küçükdeveci et al.Adaptation of the Functional Independence Measure for use in TurkeyClin Rehabil(2001)
- Mcleod JC, Ward SJ, Hicks AL. Evaluation of the Keeogo™ Dermoskeleton. Disabil Rehabil Assist Technol. 2019…
There are more references available in the full text version of this article.
[WEB] Revolutionizing Stroke Recovery: The iStride Device
Posted by Kostas Pantremenos in Assistive Technology, Gait Rehabilitation - Foot Drop, Rehabilitation robotics on January 19, 2024

The story of Maria Magdalena Valencia Juares, fondly known as Elena, is a testament to the power of innovation and the resilience of the human spirit. In 2021, Elena experienced a stroke that left her with muscle weakness and a decline in mental health, a situation further intensified by the isolation brought about by the COVID-19 pandemic. With her family residing thousands of miles away, they sought a solution to Elena’s predicament, and their answer came in the form of the iStride device. This ground-breaking tool, designed to help stroke survivors improve their walking ability, brought about incredible changes in Elena’s life, enabling her to climb stairs and dance again. The iStride device didn’t just offer her a solution; it provided hope and a renewed sense of independence.
The iStride Device: A New Hope for Stroke Survivors
The iStride device is a wearable technology designed to help stroke survivors regain their walking capability. Its real-time feedback mechanism supports users during walking exercises, leading to significant improvements in walking speed and endurance. As a result, stroke survivors gain better overall mobility and independence, as was the case with Elena.
Not only is the iStride device designed for functionality, but it is also built for comfort and adaptability. As a wearable ankle robot, it provides adjustable assistance to the user’s ankle, aiding in the regaining of strength and balance. Clinical studies corroborate the effectiveness of the iStride, with users reporting notable enhancements in their walking speed and endurance.
How the iStride Works
At its core, the iStride device is an assistive technology that aims to reduce the risk of falls in stroke survivors by providing targeted electrical stimulation to the muscles in the affected leg. This stimulation aids in improving strength and coordination, resulting in significant increases in walking speed and endurance, which in turn lead to greater independence and improved quality of life.
The iStride device employs a unique combination of visual and auditory cues to help patients improve their gait and balance. Its non-invasive nature and wearability ensure that it provides electrical stimulation to the leg muscles in a way that enhances mobility and minimises the risk of falls. The benefits of using the iStride device are not only immediate but also long-term, with studies showing sustained improvements in walking ability for stroke survivors, even one year after therapy.
iStride: Shifting the Paradigm of Stroke Recovery
The remarkable story of Elena serves as an inspiring testament to the potential of the iStride device. It is not just a rehabilitation tool; it is a device that promises a shift in the paradigm of stroke recovery. The iStride device offers a beacon of hope to stroke survivors, enabling them to regain their independence and significantly improve their quality of life.
As we move forward, it is clear that the iStride device will continue to empower stroke survivors, offering them a chance to reclaim their mobility, independence, and ultimately, their lives. Through technology and innovation, we can look forward to a future where stroke recovery is not just possible, but also achievable and sustainable.
[Abstract] Fatigue predicts level of community integration in people with stroke
Posted by Kostas Pantremenos in Fatigue, Gait Rehabilitation - Foot Drop on January 9, 2024
ABSTRACT
Background
The independent predictive power of fatigue for community integration has not been investigated, although there is an increasing amount of literature that recognizes the importance of fatigue in people with stroke.
Objectives
To examine the correlation between community integration and fatigue, walking endurance, and fear of falling; and to quantify the relative contribution of fatigue to community integration in people with stroke.
Methods
This was a cross-sectional study with 75 community-dwelling people with stroke. Data were collected using the Community Integration Measure (CIM), Fatigue Assessment Scale (FAS), 6-minute walk test (6MWT), and Survey of Activities and Fear of Falling in the Elderly (SAFE). Multiple linear regressions (forced entry method) were used to quantify the relative power of the FAS score to predict community integration in a model covering distance in the 6MWT and the SAFE score.
Results
After controlling for age, the CIM score significantly correlated with the scores for FAS (r=-0.48, p < 0.001), 6MWT distance (r = 0.24, p = 0.039), and SAFE (r=-0.39, p = 0.001). The entire model, including age, FAS score, 6MWT distance, and SAFE score, explained 26.1% of the variance in the CIM scores (F [4, 70] = 7.52, p < 0.001). The FAS scores independently explained 10.6% of the variance in the CIM scores.
Conclusions
This study suggests that fatigue is an independent predictor of community integration among people with stroke, taking into account walking endurance and fear of falling.
[ARTICLE] Robotic gait training and botulinum toxin injection improve gait in the chronic post-stroke phase: A randomized controlled trial
Posted by Kostas Pantremenos in Gait Rehabilitation - Foot Drop, Pharmacological, Rehabilitation robotics on December 23, 2023
Abstract
Background
Improving walking ability is one of the main goals of rehabilitation after stroke. When lower limb spasticity increases walking difficulty, botulinum toxin type A (BTx-A) injections can be combined with non-pharmacologic interventions such as intensive rehabilitation using a robotic approach. To the best of our knowledge, no comparisons have been made between the efficacy of robotic gait training and conventional physical therapy in combination with BTx-A injections.
Objective
To conduct a randomized controlled trial to compare the efficacy on gait of robotic gait training versus conventional physiotherapy after BTx-A injection into the spastic triceps surae in people after stroke.
Method
Thirty-three participants in the chronic stroke phase with triceps surae spasticity inducing gait impairment were included. After BTx-A injection, participants were randomized into 2 groups. Group A underwent robotic gait training (Lokomat®) for 2 weeks, followed by conventional physiotherapy for 2 weeks (n = 15) and Group B underwent the same treatment in reverse order (n = 18). The efficacy of these methods was tested using the 6-minute walk test (6MWT), comparing post-test 1 and post-test 2 with the pre-test.
Results
After the first period, the 6MWT increased significantly more in Group A than in Group B: the mean difference between the interventions was 33 m (95%CI 9; 58 p = 0.007; g = 0.95), in favor of Group A; after the second period, the 6MWT increased in both groups, but the 30 m difference between the groups still remained (95%CI 5; 55 p = 0.019; g = 0.73).
Conclusion
Two weeks of robotic gait training performed 2 weeks after BTx-A injections improved walking performance more than conventional physiotherapy. Large-scale studies are now required on the timing of robotic rehabilitation after BTx-A injection.
Introduction
Stroke is a major cause of death and disability in adults worldwide [1]. It results in spastic paresis which leads to motor impairment. Among survivors, 65% to 85% are able to walk during the first 6 months after stroke, but the functional limitations which often persist impact their quality of life [2,3]. Improving walking ability is one of the main goals of rehabilitation after stroke [4,5]. Both pharmacological and non-pharmacological treatments could be used to reduce the activity limitation caused by spastic paresis [6,7].
Intramuscular injection of botulinum toxin type A (BTx-A) performed under electromyographic or ultrasound guidance has proved to be a safe and effective means of pharmacologically reducing spasticity locally (Grade A according to the French National Health Agency). When lower limb spasticity increases individuals’ walking difficulties, BTx-A can be combined with non-pharmacological interventions as part of a multidisciplinary rehabilitation program to optimize individuals’ walking abilities [8]. Intensive rehabilitation using repetitive task training methods has been found to improve walking performance [4,9]. Robotic gait training (RGT) can be used as a repetitive, intensive, single-task method of gait training with body weight unloading and variable motor assistance, resulting in longer walking times at higher speeds [10], [11], [12]. In an international group consensus on interventions to optimize the benefits of BTx-A after stroke, Francisco et al. wrote that since repetition is important in rehabilitation, robotics are likely to gain more importance in the future [7].
Two studies have addressed the effects of combined treatment with BTx-A and robotic gait training in individuals in the chronic post-stroke phase. Picelli et al. compared the effects on spasticity of combined treatment with BTx-A and RGT with a Lokomat versus BTx-A injection alone [13]. The results showed that RGT did not enhance the antispastic effect of BTx‑A, as measured using the modified Ashworth scale and the Tardieu scale. Interestingly the latter author reported the occurrence of a significant improvement in the distance covered in the 6-minute walk test after combined treatment versus BTx-A alone. Erbil et al. observed greater benefits in terms of individuals’ walking and balance abilities after treatment with physical therapy and RGT with the Robogait as compared with physical therapy alone in individuals in the chronic post-stroke phase previously injected with BTx-A [14].
It was proposed here to test the hypothesis that RGT performed after injection of BTx-A into the triceps surae muscle would improve the walking performance of participants in the chronic post-stroke phase with spastic hemiparesis more than conventional physiotherapy (CP). The chronic phase was chosen to avoid fluctuations in performance caused by natural recovery during the first 6 months post stroke. The second argument for choosing the chronic phase was to reduce the contribution of balance disorders to walking disorders and to increase the contribution of intra-limb coordination and propulsion, which can be trained using a robotic exoskeleton [15,16].
To our knowledge, this is the first comparison of the effects of RGT and CP after BTx-A injection on gait performance in participants in the chronic post-stroke phase. The aims of the present study were as follows:
- -To compare the efficacy of RGT using the Lokomat with that of conventional physiotherapy after triceps surae BTx-A injection in participants with chronic stroke sequelae
- -To assess whether the time elapsed between BTx-A injection and RGT may contribute significantly to the gait improvements observed.
Section snippets
Materials and methods
This manuscript follows the 2017 CONSORT guidelines for reporting randomized trials evaluating non-pharmacological treatments [17].
Results
The flowchart of the results obtained is presented in Fig. 2. A total of 34 participants were enrolled and randomized into 2 groups from February 2019 to May 2021. One participant from Group A was excluded from the analysis because the investigator realized at the end of the assessments that he had been included by mistake. The analysis was based on 15 and 18 participants in Group A and Group B, respectively (Fig. 2). No significant differences were observed between the 2 groups (see Table 1).
Discussion
The aim of this study was to assess the effects of RGT treatment using a fixed exoskeleton combined with BTx-A on the walking performances of people in the chronic post-stroke phase, in comparison with CP combined with BTx-A. To our knowledge, no previous studies have addressed the question of the timing of RGT after botulinum toxin injection. The results presented here show that between W4 and W0, participants’ performances improved in the 6MWT tests more clearly after undergoing RGT than CP.
Conclusion
Our results support the hypothesis that 2 weeks of RGT with a fixed exoskeleton performed 2 weeks after BTx-A improved the walking ability of individuals in the chronic post-stroke phase more than CP. Participants with a spontaneous gait speed between 0.4 and 0.8 m/s appeared to benefit most from RGT performed between W2 and W4 after BTx-A injection. Further studies with groups paired on the basis of gait speed are now required in order to confirm this finding.
Declaration of conflicts of interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Jean Michel VITON reports financial support was provided by French government ministry of health (PHRC). Jean Michel Viton reports a relationship with Allergan France and Merz Pharma that includes support for attending meetings and travel reimbursement. Maëva COTINAT reports a relationship with Ipsen Pharma and Allergan France that includes support for attending
Funding
This study was sponsored by Assistance Publique – Hôpitaux de Marseille (DRCI).
Acknowledgement
The authors thank the participants in this study and the rehabilitation teams for their invaluable assistance, Pr. Julien Mancini for his help with statistics and Mrs. Jessica Blanc for her English writing correction.
References (34)
- P. Langhorne et al.Stroke rehabilitationLancet(2011)
- E. Allart et al.Adjunct therapies after botulinum toxin injections in spastic adults: systematic review and SOFMER recommendationsAnn Phys Rehabil Med(2022)
- G. Moucheboeuf et al.Effects of robotic gait training after stroke: a meta-analysisAnn Phys Rehabil Med(2020)
- T. Veverka et al.Cortical activity modulation by botulinum toxin type A in patients with post-stroke arm spasticity: real and imagined hand movementJ Neurol Sci(2014)
- C. Delcamp et al.Botulinum toxin combined with rehabilitation decrease corticomuscular coherence in stroke patientsClin Neurophysiol(2022)
- S.Y. Shin et al.Soft robotic exosuit augmented high intensity gait training on stroke survivors: a pilot studyJ NeuroEngineering Rehabil(2022)
- H. Hsiao et al.Control of lateral weight transfer is associated with walking speed in individuals post-strokeJ Biomech(2017)
- Johnson CO et al.Global, regional, and national burden of stroke, 1990–2016: a systematic analysis for the global burden of disease study 2016Lancet Neurol(2019)
- J. Eng et al.Gait training strategies to optimize walking ability in people with stroke: a synthesis of the evidenceExpert Rev Neurother(2007)
- D. Wade et al.Walking after stroke. Measurement and recovery over the first 3 monthsScand J Rehabil Med(1987)
View more references
[VIDEO] Post Stroke Foot Dorsiflexion: Using Electrical Stimulation to Reduce Tone & Promote Plasticity – YouTube
Posted by Kostas Pantremenos in Gait Rehabilitation - Foot Drop, Neuroplasticity, Video on December 22, 2023
Further reading on electrophysiology and muscle contractions: https://strokesciences.com/post-strok…
StrokeSciences.Com
[ARTICLE] Validating the Safe and Effective Use of a Neurorehabilitation System (InTandem) to Improve Walking in the Chronic Stroke Population: Usability Study. – Full Text
Posted by Kostas Pantremenos in Gait Rehabilitation - Foot Drop, Neuroplasticity on November 27, 2023
Abstract
Background:Persistent walking impairment following a stroke is common. Although rehabilitative interventions exist, few exist for use at home in the chronic phase of stroke recovery. InTandem (MedRhythms, Inc) is a neurorehabilitation system intended to improve walking and community ambulation in adults with chronic stroke walking impairment.
Objective:Using design best practices and human factors engineering principles, the research presented here was conducted to validate the safe and effective use of InTandem.
Methods:In total, 15 participants in the chronic phase of stroke recovery (≥6 months after stroke) participated in this validation study. Participants were scored on 8 simulated use tasks, 4 knowledge assessments, and 7 comprehension assessments in a simulated home environment. The number and types of use errors, close calls, and operational difficulties were evaluated. Analyses of task performances, participant behaviors, and follow-up interviews were conducted to determine the root cause of use errors and difficulties.
Results:During this validation study, 93% (14/15) of participants were able to successfully complete the critical tasks associated with the simulated use of the InTandem system. Following simulated use task assessments, participants’ knowledge and comprehension of the instructions for use and key safety information were evaluated. Overall, participants were able to find and correctly interpret information in the materials in order to answer the knowledge assessment questions. During the comprehension assessment, participants understood warning statements associated with critical tasks presented in the instructions for use. Across the entire study, 3 “use errors” and 1 “success with difficulty” were recorded. No adverse events, including slips, trips, or falls, occurred in this study.
Conclusions:In this validation study, people in the chronic phase of stroke recovery were able to safely and effectively use InTandem in the intended use environment. This validation study contributes to the overall understanding of residual use–related risks of InTandem in consideration of the established benefits.
Introduction
Stroke is a major cause of disability and the second leading cause of death worldwide, with its incidence and prevalence expected to increase due to an aging population [1,2]. Many people in the chronic phase of stroke recovery (commonly defined as ≥6 months after stroke) experience walking impairment [3] and consider the ability to walk in their community as “either essential or very important” [4]. Walking rehabilitation can positively impact the well-being of stroke survivors and their families. It can also restore independence—a prospective study in the chronic stroke population reported that better walking ability was positively correlated with quality of life and the ability to live independently [5]. Clinical practice guidelines recommend various interventions for walking impairment, including physical therapy and braces such as an ankle foot orthosis [6–8]. Rhythmic auditory stimulation (RAS) is another clinically effective intervention for the rehabilitation of movements that are naturally rhythmic (eg, walking) [9]. RAS draws on a naturally occurring phenomenon called auditory-motor entrainment. During entrainment, an external auditory rhythm enables subconscious synchronization between the auditory and motor systems to drive coordinated movement patterns [10,11]. RAS has shown clinical benefits related to walking for patients with stroke across the subacute and chronic phases in many studies, several of which are randomized controlled trials (RCTs) [12–18]. In particular, speed, step length, cadence, balance, and dynamic postural stability [12,19,20] have been shown to improve in people who have had a stroke and receive RAS. In addition, the US Department of Veterans Affairs incorporated rhythmic auditory cueing into its clinical practice guidelines for the management of stroke rehabilitation in 2019 [21].
Currently, clinicians administer the RAS protocol in rehabilitation hospitals or clinics, while accessible at-home RAS-based interventions are nonexistent. Rehabilitation at home can carry benefits including half the risk of readmission, lower caregiver strain [22], reduced cost, and greater patient satisfaction [23] relative to hospital rehabilitation. For those in the chronic phase of stroke recovery, physical therapy and to a greater extent RAS can be difficult to access due to limited insurance coverage and the limited number of neurologic music therapists who deliver RAS. The lack of a solution for at-home walking rehabilitation is a critical gap in chronic stroke recovery, and it is imperative that solutions that are safe and effective to use are developed and delivered to address this unmet need.
To help close this gap, MedRhythms has designed MR-001 (InTandem, MedRhythms, Inc), a neurorehabilitation system that delivers a RAS-based intervention for chronic stroke walking impairment and is intended to be used independently at home. […]

