Posts Tagged UE
[Abstract] Neurostimulation for treatment of post-stroke impairments
Posted by Kostas Pantremenos in Epilepsy on April 8, 2024
Abstract
Neurostimulation, the use of electrical stimulation to modulate the activity of the nervous system, is now commonly used for the treatment of chronic pain, movement disorders and epilepsy. Many neurostimulation techniques have now shown promise for the treatment of physical impairments in people with stroke. In 2021, vagus nerve stimulation was approved by the FDA as an adjunct to intensive rehabilitation therapy for the treatment of chronic upper extremity deficits after ischaemic stroke. In 2024, pharyngeal electrical stimulation was conditionally approved by the UK National Institute for Health and Care Excellence for neurogenic dysphagia in people with stroke who have a tracheostomy. Many other approaches have also been tested in pivotal device trials and a number of approaches are in early-phase study. Typically, neurostimulation techniques aim to increase neuroplasticity in response to training and rehabilitation, although the putative mechanisms of action differ and are not fully understood. Neurostimulation techniques offer a number of practical advantages for use after stroke, such as precise dosing and timing, but can be invasive and costly to implement. This Review focuses on neurostimulation techniques that are now in clinical use or that have reached the stage of pivotal trials and show considerable promise for the treatment of post-stroke impairments.
Key points
- Neurostimulation techniques are ideally suited for use during stroke recovery owing to their ability to target anatomical structures or neuronal networks, alongside precise timing and dosing.
- Paired invasive vagus nerve stimulation has been shown to increase the number of people who achieve clinically important improvements in upper extremity impairment and performance of functional tasks following stroke. The treatment is now in clinical use in the USA.
- Several other neurostimulation techniques show promise for post-stroke impairments but definitive data from adequately powered trials are lacking.
- Pharyngeal electrical stimulation increases the odds of decannulation following tracheostomy and is under investigation as a treatment for post-stroke dysphagia.
[ARTICLE] Feasibility of a Self-directed Upper Extremity Training Program to Promote Actual Arm Use for Individuals Living in the Community With Chronic Stroke – Full Text
Posted by Kostas Pantremenos in Paretic Hand on March 1, 2024
Highlights
- Feasibility of a self-directed upper extremity training program to promote actual arm use for individuals living in the community with chronic stroke
-
- •Distinct from clinic-based rehabilitation, self-directed rehabilitation approaches must address unique challenges related to decreased client motivation and adherence.
- •Shared decision making and behavior change frameworks can support the implementation of UE self-directed rehabilitation.
- •The Use My Arm-Remote program was feasible and safe to implement for individuals living in the community with chronic stroke.
Abstract
Objective
To determine the feasibility of a self-directed training protocol to promote actual arm use in everyday life. The secondary aim was to explore the initial efficacy on upper extremity (UE) outcome measures.
Design
Feasibility study using multiple methods.
Setting
Home and outpatient research lab.
Participants
Fifteen adults (6 women, 9 men, mean age=53.08 years) with chronic stroke living in the community. There was wide range of UE functional levels, ranging from dependent stabilizer (limited function) to functional assist (high function).
Intervention
Use My Arm-Remote protocol. Phase 1 consisted of clinician training on motivational interviewing (MI). Phase 2 consisted of MI sessions with participants to determine participant generated goals, training activities, and training schedules. Phase 3 consisted of UE task-oriented training (60 minutes/day, 5 days/week, for 4 weeks). Participants received daily surveys through an app to monitor arm training behavior and weekly virtual check-ins with clinicians to problem-solve challenges and adjust treatment plans.
Outcome Measures
Primary outcome measures were feasibility domains after intervention, measured by quantitative study data and qualitative semi-structured interviews. Secondary outcomes included the Canadian Occupational Performance Measure (COPM), Motor Activity Log (MAL), Fugl-Meyer Assessment (FMA), and accelerometry-based duration of use metric measured at baseline, discharge, and 4-week follow-up.
Results
The UMA-R was feasible in the following domains: recruitment rate, retention rate, intervention acceptance, intervention delivery, adherence frequency, and safety. Adherence to duration of daily practice did not meet our criteria. Improvements in UE outcomes were achieved at discharge and maintained at follow-up as measured by COPM-Performance subscale (F[1.42, 19.83]=17.72, P<.001) and COPM-Satisfaction subscale (F[2, 28]=14.73, P<.001), MAL (F[1.31, 18.30]=12.05, P<.01) and the FMA (F[2, 28]=16.62, P<.001).
Conclusion
The UMA-R was feasible and safe to implement for individuals living in the community with chronic stroke. Adherence duration was identified as area of refinement. Participants demonstrated improvements in standardized UE outcomes to support initial efficacy of the UMA-R. Shared decision-making and behavior change frameworks can support the implementation of UE self-directed rehabilitation. Our results warrant the refinement and further testing of the UMA-R.
Stroke is the leading cause of disability in adults in the United States.1,2 Approximately 50% of stroke survivors experience upper extremity (UE) impairment,3,4 and of those, over 60% have continued impairment after 6 months.5,6 Based on the International Classification of Functioning, Disability, and Health, impairment is defined as limitations in body function and structures.7 Limited UE functional use after stroke can affect performance with activities of daily living, return to work, and quality of life.8, 9, 10 Chronic UE disability and subsequent effect on participation and independence highlights the need for continued therapy beyond acute and subacute periods of recovery as individuals re-integrate back into the community.11
Evidence suggests that UE improvements achieved in the clinic do not automatically generalize to actual use of the affected UE in the home and community.12, 13, 14, 15, 16 In other words, what clients do in the clinic is different from what they do at home. Actual arm use, defined as the use of the affected UE in everyday activities in real world settings,17 was first introduced into the stroke literature as a key outcome for Constraint Induced Movement Therapy (CIMT) measured by the Motor Activity Log (MAL). Despite the widespread research on CIMT, actual arm use as a construct has been largely understudied. Distinct from motor capacity or functional ability, emerging literature suggests that actual arm use is a complex behavior informed by multiple factors related to the person, their environment, and the task itself.18 Therefore, interventional approaches that target actual arm use likely require self-directed training in daily activities completed in real-world settings.19
We define self-directed rehabilitation as structured motor or functional training program with clear patient objectives completed remotely in the client’s home under indirect supervision of a clinician.20,21 Evidence comparing the effectiveness of home-based vs clinic-based rehabilitation has consistently demonstrated no differences in physical and motor outcomes for older adult populations with general chronic health conditions.20,22, 23, 24 Similarly, home-based UE interventions in stroke are comparable with clinic-based interventions for UE activity performance and dexterity.25 Self-directed training approaches are also effective in post stroke rehabilitation. There is a wide range of self-directed training approaches with studies using robotics, interactive gaming, CIMT, and electrical stimulation (e-stim) with CIMT and e-stim approaches demonstrating most robust improvements in UE functional ability and daily use.26 While CIMT is the most established self-directed training approach, common limitations include the narrow inclusion criteria 27,28 and limited ecological validity of constraining the less affected hand because individuals complete most daily tasks bilaterally.29,30 Additionally, no differences were reported between structured vs non-structured approaches within self-directed rehabilitation studies.19 The literature on the effectiveness of self-directed UE rehabilitation in stroke is emerging and the available evidence is mixed.19,24 More work is needed in this area to delineate key components of self-directed UE rehabilitation that effectively maximize actual arm use in daily life.
To address the need to better understand and develop effective home-based, self-directed rehabilitation approaches, Use My Arm-Remote (UMA-R) was adapted with permission from the original Use My Arm.31,32 UMA-R is a self-directed rehabilitation program informed by both motor learning principles and health behavior change frameworks. UMA-R combines several evidence-based approaches (eg, task-oriented practice, motivational interviewing (MI), ecological momentary assessments [EMAs]) to target actual arm use. Distinct from clinic-based rehabilitation, self-directed rehabilitation in the home setting must address unique challenges related to decreased motivation and adherence, because clients must self-initiate training and complete the recommended tasks with indirect therapist supervision. UMA-R uses shared decision-making (SDM) approach and EMAs to maximize intrinsic motivation and adherence.
The task-oriented approach, also referred to as task specific training in the literature, is based on the principles of neuroplasticity and the systems model of motor control.33 Task-oriented approach emphasizes the importance of selecting tasks that are meaningful to the client, training in real-world environments outside of therapy settings, and the use of motor learning principles (eg, repetition and practice, adaptation).33 UE training using a task-oriented approach can be implemented by having clients select and practice meaningful everyday tasks that require UE use. Emphasis is placed on using the client’s own materials within the context of daily routines (eg, practicing brushing their hair using their own brush during their self-care routine).
SDM is a collaborative process between patients, families, and clinicians when patient values and preferences are prioritized along with existing evidence when making decisions about a specific scenario.34 MI can be used to implement an SDM approach. MI is a counseling approach based on the guiding principle that behavior change is contingent on the client- rather than the clinician-driving decision and expressing the reasons for change.35 MI has been integrated into cognitive and behavioral interventions to maximize self-initiated action and encourage activity engagement.35
EMA produces real-time data about an individual’s behavior, mood, or experiences in their natural settings.36,37 EMA has been used extensively to target health behavior change such as smoking cessation, diabetes management, and weight loss.36 EMA in stroke has been used to measure caregiver burden,38 predictors of depression,39,40 and fatigue.41 The emergence and use of mobile technologies (ie, text messages or phone app) has greatly increased the ability to implement EMA successfully in a variety of clinical populations and is well-suited to capture client’s daily activities in the context of their routines at home.
UMA-R addresses the unique challenges for self-directed UE training and bridges the gap between gains made in the clinic and generalized arm use in real world settings. As an initial step in intervention development and testing, the primary aim of the study was to examine the feasibility of the UMA-R protocol by addressing the following domains: recruitment and retention rates, intervention delivery, intervention acceptance, intervention adherence, and safety. The secondary aim of the study was to report initial efficacy of the UMA-R as measured by UE outcomes. […]

[Abstract] A Review on Soft Exoskeletons for Hand Rehabilitation
Posted by Kostas Pantremenos in Paretic Hand, Rehabilitation robotics on February 24, 2024
Background: How to enhance the quality of life for the elderly has emerged as a key issue in many nations due to the ageing population. Stroke is the most prevalent disease among the elderly; specifically, the hand dysfunction caused by stroke is also a powerful obstacle to the daily life of the elderly. Soft Exoskeletons for Hand Rehabilitation (SEHRs) have become a major trend for the future due to the increasing demand for hand rehabilitation.
Objective: To provide a reference for readers in this field by introducing the most recent research developments in the field of SEHR, including their classification and properties.
Methods: By reviewing different types of hand rehabilitation exoskeleton research papers and patents, the advantages and disadvantages, differences, and applications of various SEHRs were summarized.
Results: According to the driving mode and realizing the function of SEHRs, the structure characteristics of SEHRs are analyzed and compared. The key problems and future development trends of SEHRs were expounded.
Conclusion: According to the driving method, the research shows that SEHRs can be divided into Air-Driven Soft Exoskeletons for Hand Rehabilitation (ADSEHRs), Motor-Driven Soft Exoskeletons for Hand Rehabilitation (MDSEHRs), and Hybrid-Driven Soft Exoskeletons for Hand Rehabilitation (HDSEHRs). Future research is required to further optimize the flexibility and adaptability of soft exoskeletons, improve their accuracy and sensitivity, and enhance human-machine interaction with the human hand.
[Abstract + References] Research progress and development trend of flexible hand rehabilitation gloves
Posted by Kostas Pantremenos in Paretic Hand, REHABILITATION, Rehabilitation robotics on February 20, 2024
Abstract
The flexible hand rehabilitation glove is proposed to solve the problems of long rehabilitation training time for patients and high workload for doctors, and to make the treatment more effective. With the advances in robotics, robotic-assisted therapy has developed rapidly and has become an essential complement to conventional treatment. To understand flexible hand rehabilitation glove devices, with the different construction types of actuators and drive methods as the mainline, the corresponding study of these structures as the auxiliary lines. The characteristics and the current state of research have been discussed. A brief introduction to manufacturing actuators and rehabilitation systems is also given. Through the analysis of hand rehabilitation gloves, some current advantages and disadvantages are summarized, and future directions and functional diversification are envisaged. Certain feasible research suggestions have been proposed for future development regarding structure, functional diversity, and a combination of driving methods. These include that there will be more combinations of pneumatic and motor driven, combining the advantages of both methods to overcome the disadvantages of each. The structural design will be more in line with anatomy and ergonomics, make it more esthetically pleasing. More innovative controls methods will be adopted to achieve more complex rehabilitation functions.
References
1. Taylor CL, Schwarz RJ. The anatomy and mechanics of the human hand. Artif Limbs 1955; 2(2): 22–35.
2. Chim H. Hand and wrist anatomy and biomechanics: a comprehensive guide. Plast Reconstr Surg 2017; 140(4): 865–865.
3. Nas K, Yazmalar L, Şah V, et al. Rehabilitation of spinal cord injuries. World J Orthop 2015; 6(1): 8–16.
4. Silva NA, Sousa N, Reis RL, et al. From basics to clinical: a comprehensive review on spinal cord injury. Prog Neurobiol 2014; 114: 25–57.
5. Davis A. Medical, psychosocial and vocational aspects of disability, 4th edition.J Rehabil 2015; 81(3): 58–59.
6. Gassert R, Dietz V. Rehabilitation robots for the treatment of sensorimotor deficits: a neurophysiological perspective. J Neuroeng Rehabil 2018; 15(1): 46.
7. Mlinac ME, Feng MC. Assessment of activities of daily living, self-care, and independence. Arch Clin Neuropsychol 2016; 31(6): 506–516.
8. Rudd AG, Bowen A, Yang GR. The latest national clinical guideline for stroke. Clin Med 2017; 17(4): 382–383.
9. O’Driscoll SW, Giori NJ. Continuous passive motion (CPM): theory and principles of clinical application. J Rehabil Res Dev 2000; 37(2): 179–188.
10. Bütefisch C, Hummelsheim H, Denzler P, et al. Repetitive training of isolated movements improves the outcome of motor rehabilitation of the centrally paretic hand. J Neurol Sci 1995; 130(1): 59–68.
11. Iqbal J, Baizid K. Stroke rehabilitation using exoskeleton-based robotic exercisers: mini review. Biomed Res 2014; 26(26): 197–201.
12. Rotella M, Reuther K, Hofmann C, et al. An orthotic hand-assistive exoskeleton for actuated pinch and grasp. In: 2009 IEEE 35th annual northeast bioengineering conference, Cambridge, MA, 3–5 April 2009, pp.338–339. New York: IEEE.
13. Li G, Cheng L, Gao Z, et al. Development of an untethered adaptive thumb exoskeleton for delicate rehabilitation assistance. IEEE Trans Robot 2022; 38(6): 3514–3529.
14. Kang B, In H, Cho K. Modeling of tendon driven soft wearable robot for the finger. In: 2013 10th international conference on ubiquitous robots and ambient intelligence (URAI), Jeju, South Korea, 30 October–2 November 2013, pp.459–460. New York: IEEE.
15. Yap HK, Lim JH, Goh JCH, et al. Design of a soft robotic glove for hand rehabilitation of stroke patients with clenched fist deformity using inflatable plastic actuators. J Med Device 2016; 10(4): 044504.
16. Yap HK, Lim JH, Nasrallah F, et al. Characterisation and evaluation of soft elastomeric actuators for hand assistive and rehabilitation applications. J Mar Eng Technol 2016; 40(4): 199–209.
17. Shahid T, Gouwanda D, Nurzaman SG, et al. Moving toward soft robotics: a decade review of the design of hand exoskeletons. Biomimetics 2018; 3(3): 17.
18. Ahmed Y, Ai-Neami, Lateef S. Robotic glove for rehabilitation purpose: review. In: The 3rd scientific conference of electrical and electronic engineering researches (SCEEER), Basrah, Iraq, 15–16 June 2020, pp.15–16. New York: IEEE.
19. du Plessis T, Djouani K, Oosthuizen C. A review of active hand exoskeletons for rehabilitation and assistance. Robotics 2021; 10(1): 40.
20. Tran P, Jeong S, Herrin KR, et al. Review: hand exoskeleton systems, clinical rehabilitation practices, and future prospects. IEEE Trans Med Robot Bionics 2021; 3(3): 606–622.
21. Pérez Vidal AF, Rumbo Morales JY, Ortiz Torres G, et al. Soft exoskeletons: development, requirements, and challenges of the last decade. Actuators 2021; 10(7): 166.
22. Tiboni M, Amici C. Soft gloves: a review on recent developments in actuation, sensing, control and applications. Actuators 2022; 11(8): 232.
23. Heo P, Gu GM, Lee SJ, et al. Current hand exoskeleton technologies for rehabilitation and assistive engineering. Int J Precis Eng Manuf 2012; 13(5): 807–824.
24. Balasubramanian S, Klein J, Burdet E. Robot-assisted rehabilitation of hand function. Curr Opin Neurol 2010; 23(6): 661–670.
25. Fonseca MCR, Elui VMC, Lalone E, et al. Functional, motor, and sensory assessment instruments upon nerve repair in adult hands: systematic review of psychometric properties. Syst Rev 2018; 7(1): 175.
26. Pataky TC, Latash ML, Zatsiorsky VM. Multifinger Ab- and adduction strength and coordination. J Hand Ther 2008; 21(4): 377–385.
27. Hunter S, Crome P. Hand function and stroke. Rev Clin Gerontol 2002; 12(1): 68–81.
28. Boser QA, Dawson MR, Schofield JS, et al. Defining the design requirements for an assistive powered hand exoskeleton: a pilot explorative interview study and case series. Prosthet Orthot Int 2021; 45(2): 161–169.
29. Aubin PM, Sallum H, Walsh C, et al. A pediatric robotic thumb exoskeleton for at-home rehabilitation: the isolated orthosis for thumb actuation (IOTA). Int J Intell Comput Cybern 2014; 7(3): 233–252.
30. Hines AE, Crago PE, Billian C. Hand opening by electrical stimulation in patients with spastic hemiplegia. IEEE Trans Rehabil Eng 1995; 3(2): 193–205.
31. Ilievski F, Mazzeo AD, Shepherd RF, et al. Soft robotics for chemists. Angew Chem 2011; 50(8): 1890–1935.
32. Polygerinos P, Lyne S, Wang Z, et al. Towards a soft pneumatic glove for hand rehabilitation. In: 2013 IEEE/RSJ international workshop on intelligent robots and systems (IROS), Tokyo, Japan, 3–7 November 2013, pp.1512–1517. New York: IEEE.
33. Tiboni M, Loda D. Monolithic PneuNets soft actuators for robotic rehabilitation: methodologies for design, production and characterization. Actuators 2023; 12(7): 299.
34. Yang Y, Chen Y, Li Y, et al. Bioinspired robotic fingers based on pneumatic actuator and 3D printing of smart material. Soft Robot 2017; 4(2): 147–162.
35. Yap H, Lim J, Nasrallah F, et al. A soft exoskeleton for hand assistive and rehabilitation application using pneumatic actuators with variable stiffness. In: 2015 IEEE international conference on robotics and automation (ICRA), Seattle, WA, 26–30 May 2015, pp.4967–4972. New York: IEEE.
36. Wang J, Fei Y, Pang W. Design, modeling, and testing of a soft pneumatic glove with segmented pneunets bending actuators. IEEE/ASME Trans Mechatron 2019; 24(3): 990–1001.
37. Guo S, Zhao F, Wei W, et al. Soft actuator for hand rehabilitation. In: 2015 IEEE international conference on mechatronics and automation (ICMA), Beijing, China, 2–5 August 2015. New York: IEEE.
38. Mosadegh B, Polygerinos P, Keplinger C, et al. Pneumatic networks for soft robotics that actuate rapidly. Adv Funct Mater 2014; 24(15): 2163–2170.
39. Lalegani Dezaki M, Bodaghi M, Serjouei A, et al. Soft pneumatic actuators with controllable stiffness by bio-inspired lattice chambers and fused deposition modeling 3D printing. Adv Eng Mater 2023; 25(6): 2200797.
40. Li X, Lin Y, Jia J. Design and implementation of a hand rehabilitation device for stroke. Mech Eng Technol 2021; 50(3): 104–105.
41. Jiang Y, Chen D, Liu P, et al. Fishbone-inspired soft robotic glove for hand rehabilitation with multi-degrees-of-freedom. In: 2018 IEEE international conference on soft robotics (RoboSoft), Livorno, Italy, 24–28 April 2018, pp.394–399. New York: IEEE.
42. Meng F, Liu C, Li Y, et al. Personalized and safe soft glove for rehabilitation training. Electronics 2023; 12(11): 2531.
43. Zhang J, Hu D, Hong J, et al. A user-defined passive pneumatic soft body manipulator. CN Patent 110 236 880. September17, 2019.
44. Haghshenas-Jaryani M, Patterson RM, Bugnariu N, et al. A pilot study on the design and validation of a hybrid exoskeleton robotic device for hand rehabilitation. J Hand Ther 2020; 33(2): 198–208.
45. Fan S, Jin M, Li B, et al. Rigid-flexible coupled extensible articulated soft exoskeleton glove and method. CN Patent 111 150 602, 15 May 2020.
46. Yang K, Mao Z, Yamamoto K. Finger joint rehabilitation device. US Patent 10 751 244, 25 August 2020.
47. Yang R, Shi H, Yuan L, et al. A wearable soft hand functional rehabilitation glove. CN Patent 111 821 140, 27 October 2020.
48. Liu D, Wang M, Bi C, et al. A wearable finger rehabilitation device with a combination of rigid and soft activation and its manufacturing method. CN Patent 110 772 402, 11 February 2020.
49. Hu D, Zhang J, Yang Y, et al. A novel soft robotic glove with positive-negative pneumatic actuator for hand rehabilitation. In: 2020 IEEE/ASME international conference on advanced intelligent mechatronics (AIM), Boston, MA, 6–9 July 2020. New York: IEEE.
50. Yun SS, Kang BB, Cho KJ. Exo-Glove PM: an easily customizable modularized pneumatic assistive glove. IEEE Robot Autom Lett 2017; 2(3): 1725–1732.
51. Heung KHL, Tong RKY, Lau ATH, et al. Robotic glove with soft-elastic composite actuators for assisting activities of daily living. Soft Robot 2019; 6(2): 289–304.
52. Heung K, Tong Z, Ho L, et al. Design of a 3D printed soft robotic hand for stroke rehabilitation and daily activities assistance. In: 2019 IEEE 16th international conference on rehabilitation robotics (ICORR), Toronto, ON, 24–28 June 2019, pp.65–70. New York: IEEE.
53. Connolly F, Polygerinos P, Walsh CJ, et al. Mechanical programming of soft actuators by varying fiber angle. Soft Robot 2015; 2(1): 26–32.
54. Polygerinos P, Wang Z, Overvelde JTB, et al. Modeling of soft fiber-reinforced bending actuators. IEEE Trans Robot 2015; 31(3): 778–789.
55. Wang B, McDaid A, Aw K, et al. Design and development of a skinny bidirectional soft glove for post-stroke hand rehabilitation. In: 2017 intelligent systems conference (IntelliSys), London, 7–8 September 2017, pp.979–987. New York: IEEE.
56. Ramos O, Múnera M, Moazen M, et al. Assessment of soft actuators for hand exoskeletons: pleated textile actuators and fiber-reinforced silicone actuators. Front Bioeng Biotechnol 2022; 10: 924888.
57. Lin M, Paul R, Abd M, et al. Feeling the beat: a smart hand exoskeleton for learning to play musical instruments. Front Rob AI 2023; 10: 1212768.
58. Wang Y, Kokubu S, Zhou Z, et al. Designing soft pneumatic actuators for thumb movements. IEEE Robot Autom Lett 2021; 6(4): 8450–8457.
59. Tarvainen T, Fernandez-Vargas J, Yu W. New layouts of fiber reinforcements to enable full finger motion assist with pneumatic multi-chamber elastomer actuators. Actuators 2018; 7(2): 31.
60. Meng M. A hand-assisted rehabilitation drive. CN Patent 110 840 706, 28 February 2020.
61. Cao X, Ma K, Jiang Z, et al. A soft robotic glove for hand rehabilitation using pneumatic actuators with jamming structure. In: 2021 40th Chinese control conference (CCC), Shanghai, China, 26-28 July 2021. New York: IEEE.
62. Zhang H, Kumar AS, Chen F, et al. Topology optimized multimaterial soft fingers for applications on grippers, rehabilitation, and artificial hands. IEEE/ASME Trans Mechatron 2019; 24(1): 120–131.
63. Meng M. Structural design of a soft hand functional rehabilitation robot glove. CN Patent 112 999 014, 22 June 2021.
64. Kladovasilakis N, Kostavelis I, Sideridis P, et al. A novel soft robotic exoskeleton system for hand rehabilitation and assistance purposes. Appl Sci 2022; 13(1): 553.
65. Meng N, Kun W, Mingxin L, et al. Design, analysis and experiment of finger soft actuator with nested structure for rehabilitation training. Adv Mech Eng 2020; 12(11): 1–15.
66. Sun ZS, Guo ZH, Tang W. Design of wearable hand rehabilitation glove with soft hoop-reinforced pneumatic actuator. J Central South Univ 2019; 26: 106–119.
67. Polygerinos P, Wang Z, Galloway KC, et al. Soft robotic glove for combined assistance and at-home rehabilitation. Robot Auton Syst 2015; 73: 135–143.
68. Li Z, Lv H, Li Q. An articulated soft body rehabilitation robot glove. CN Patent 113 332 104, 3 September 2021.
69. Gaylord R. Fluid actuated motor system and stroking device. US Patent 2 844 126, 22 July 1958.
70. Daerden F, Lefeber D. Pneumatic artificial muscles: actuators for robotics and automation. Eur J Mech Env Eng 2002; 47: 11–21.
71. Baldwin HA. Realizable models of muscle function. In Biomechanics: Proceedings of the first rock island arsenal biomechanics symposium april 5–6, 1967. Springer, New York, 1969, pp.139–147.
72. Andrikopoulos G, Nikolakopoulos G, Manesis S. Pneumatic artificial muscles: a switching model predictive control approach. Control Eng Pract 2013; 21(12): 1653–1664.
73. Andrikopoulos G, Nikolakopoulos G, Manesis S. Design and development of an exoskeletal wrist prototype via pneumatic artificial muscles. Meccanica 2015; 50(11): 2709–2730.
74. Takosoglu JE, Laski PA, Blasiak S, et al. Determining the static characteristics of pneumatic muscles. Meas Control 2016; 49(2): 62–71.
75. Al-Fahaam H, Davis S, Nefti-Meziani S. The design and mathematical modelling of novel extensor bending pneumatic artificial muscles (EBPAMs) for soft exoskeletons. Robot Auton Syst 2018; 99: 63–74.
76. Peng G, Fan X, Liu X, et al. Design and control of flexible wearable rehabilitation gloves. Med Biomech 2019; 34(6): 637–643.
77. Takahashi N, Furuya S, Koike H. Soft exoskeleton glove with human anatomical architecture: production of dexterous finger movements and skillful piano performance. IEEE Trans Haptics 2020; 13(4): 679–690.
78. Wang J, Liu Z, Fei Y. Design and testing of a soft rehabilitation glove integrating finger and wrist function. J Mech Robot 2019; 11(1): 011015.
79. Maldonado-Mejía JC, Múnera M, Diaz CAR, et al. A fabric-based soft hand exoskeleton for assistance: the exhand exoskeleton. Front Neurorobot 2023; 17: 1091827.
80. Elmoughni HM, Yilmaz AF, Ozlem K, et al. Machine-knitted seamless pneumatic actuators for soft robotics: design, fabrication, and characterization. Actuators 2021; 10(5): 94.
81. Yap HK, Khin PM, Koh TH, et al. A fully fabric-based bidirectional soft robotic glove for assistance and rehabilitation of hand impaired patients. IEEE Robot Autom Lett 2017; 2(3): 1383–1390.
82. Feng M, Yang D, Gu G. High-force fabric-based pneumatic actuators with asymmetric chambers and interference-reinforced structure for soft wearable assistive gloves. IEEE Robot Autom Lett 2021; 6(2): 3105–3111.
83. Ge L, Chen F, Wang D, et al. Design, modeling, and evaluation of fabric-based pneumatic actuators for soft wearable assistive gloves. Soft Robot 2020; 7(5): 583–596.
84. Jeong U, In HK, Cho KJ. Implementation of various control algorithms for hand rehabilitation exercise using wearable robotic hand. Intell Serv Robot 2013; 6(4): 181–189.
85. Aoun A, Lliovits A, Kassem A, et al. Arthro-Glove a hybrid bionic glove for patients diagnosed with arthritis, als and/or dysmorphia. In: 2018 9th Cairo international biomedical engineering conference (CIBEC), Cairo, Egypt, 20–22 December 2018, pp.106–109. New York: IEEE.
86. Xu D, Wu Q, Zhu Y. Development of a soft cable-driven hand exoskeleton for assisted rehabilitation training. Ind Rob 2021; 48(2): 189–198.
87. Alnajjar F, Umari H, Ahmed WK, et al. CHAD: compact hand-assistive device for enhancement of function in hand impairments. Robot Auton Syst 2021; 142: 103784.
88. Biggar S, Yao W. Design and evaluation of a soft and wearable robotic glove for hand rehabilitation. IEEE Trans Neural Syst Rehabil Eng 2016; 24(10): 1071–1080.
89. Serrano D, Copaci D, Arias J, et al. SMA-based soft exo-glove. IEEE Robot Autom Lett 2023; 8(9): 5448–5455.
90. Abdelhafiz MH, Andreasen Struijk LNS, Dosen S, et al. Biomimetic tendon-based mechanism for finger flexion and extension in a soft hand exoskeleton: design and experimental assessment. Sensors 2023; 23(4): 2272.
91. Chen W, Li G, Li N, et al. Soft exoskeleton with fully actuated thumb movements for grasping assistance. IEEE Trans Robot 2022; 38(4): 2194–2207.
92. Tran P, Jeong S, Lyu F, et al. FLEXotendon Glove-iii: voice-controlled soft robotic hand exoskeleton with novel fabrication method and admittance grasping control. IEEE/ASME Trans Mechatron 2022; 27(5): 3920–3931.
93. Kang B, Lee H, In H, et al. Development of a polymer-based tendon-driven wearable robotic hand. In: 2016 IEEE International Conference on Robotics and Automation (ICRA), Stockholm, 16–21 May 2016, pp.3750–3755. New York: IEEE.
94. Kin D, Heo S, Park H. Biomimetic finger extension mechanism for soft wearable hand rehabilitation devices. In: 2017 international conference on rehabilitation robotics (ICORR), London, 17–20 July 2017, pp.1326–1330. New York: IEEE.
95. Setiawan JD, Ariyanto M, Nugroho S, et al. Fuzzy logic control for a soft exoskeleton glove using a motor-tendon actuator. Ing Invest 2021; 41(1): e81531.
96. Tang D, Lv X, Qi L, et al. A tendon wire driven exoskeleton for functional hand rehabilitation. CN Patent 115 284 261, 4 November 2022.
97. Mohammadi A, Lavranos J, Choong P, et al. Flexo-glove: a 3D printed soft exoskeleton robotic glove for impaired hand rehabilitation and assistance. In: 2018 40th annual international conference of the IEEE engineering in medicine and biology society (EMBC), Honolulu, HI, 18–21 July 2018, pp.2120–2123. New York: IEEE.
98. Liu A, Qiu J, Wang F, et al. An adaptive and jointless hand exoskeleton system design. In: 2018 15th international conference on control, automation, robotics and vision (ICARCV), Singapore, 18–21 November 2018, pp.579–584. New York: IEEE.
99. Arata J, Ohmoto K, Gassert R, et al. A new hand exoskeleton device for rehabilitation using a three-layered sliding spring mechanism. In: 2013 IEEE international conference on robotics and automation, Karlsruhe, Germany, 6–10 May 2013, pp.3902–3907. New York: IEEE.
100. Nazari V, Pouladian M, Zheng YP, et al. A compact and lightweight rehabilitative exoskeleton to restore grasping functions for people with hand paralysis. Sensors 2021; 21(20): 6900.
101. Lin L, Zhang F, Yang L, et al. Design and modeling of a hybrid soft-rigid hand exoskeleton for poststroke rehabilitation. Int J Mech Sci 2021; 212: 106831.
102. Bützer T, Lambercy O, Arata J, et al. Fully wearable actuated soft exoskeleton for grasping assistance in everyday activities. Soft Robot 2021; 8(2): 128–143.
103. Gerez L, Gao G, Dwivedi A, et al. A hybrid, wearable exoskeleton glove equipped with variable stiffness joints, abduction capabilities, and a telescopic thumb. IEEE Access 2020; 8: 173345–173358.
104. Stilli A, Cremoni A, Bianchi M, et al. Airexglove – a novel pneumatic exoskeleton glove for adaptive hand rehabilitation in post-stroke patients. In: 2018 IEEE international conference on soft robotics (RoboSoft), Livorno, Italy, 24–28 April 2018, pp.579–584. New York: IEEE.
105. Tang D, Qi L, Shen C, et al. A flexible hand rehabilitation exoskeleton and its control method. CN Patent 116 650 285, 29 August 2023.
106. Cho KJ, Koh JS, Kim S, et al. Review of manufacturing processes for soft biomimetic robots. Int J Precis Eng Manuf 2009; 10: 171–181.
107. Yap H, Goh J, Yeow R. Design and characterization of soft actuator for hand rehabilitation application. In: 6th European conference of the international federation for medical and biological engineering, Dubrovnik, Croatia, 7–11 September 2014, vol. 45, pp.367–370.
108. Yirmibesoglu O, Menguc Y. Hybrid soft sensor with embedded imus to measure motion. In: 2016 IEEE international conference on automation science and engineering (CASE), Fort Worth, TX, 21–25 August 2016, pp.798–804. New York: IEEE.
109. Sun Y, Song S, Liang X, et al. A miniature soft robotic manipulator based on novel fabrication methods. IEEE Robot Autom Lett 2016; 1(2): 617–623.
110. Gong Z, Xie Z, Yang X, et al. Design, fabrication and kinematic modeling of a 3D-motion soft robotic arm. In: 2016 IEEE international conference on robotics and biomimetics (ROBIO), Qingdao, China, 3–7 December 2016, pp.509–514. New York IEEE.
111. Wallin TJ, Pikul J, Shepherd RF. 3D printing of soft robotic systems. Nat Rev Mater 2018; 3: 84–100.
112. Hinton TJ, Jallerat Q, Palchesko RN, et al. Three-dimensional printing of complex biological structures by freeform reversible embedding of suspended hydrogels. Sci Adv 2015; 1(9): e1500758.
113. Nyberg EL, Farris AL, Hung BP, et al. 3D-printing technologies for craniofacial rehabilitation, reconstruction, and regeneration. Ann Biomed Eng 2017; 45(1): 45–57.
114. Wolf SL, Blanton S, Baer H, et al. Repetitive task practice: a critical review of constraint-induced movement therapy in stroke. Neurologist 2002; 8(6): 325–338.
115. Altschuler EL, Wisdom SB, Stone L, et al. Rehabilitation of hemiparesis after stroke with a mirror. Lancet 1999; 353(9169): 2035–2036.
116. Boos A, Qiu Q, Fluet G, et al. Haptically facilitated bimanual training combined with augmented visual feedback in moderate to severe hemiplegia. In: 2011 annual international conference of the IEEE engineering in medicine and biology society, Boston, MA, 30 August–3 September 2011, pp.3111–3114. New York: IEEE.
117. Kim SY, Kim YY. Mirror therapy for phantom limb pain. Korean J Pain 2012; 25(4): 272–274.
118. Chen X, Gong L, Wei L, et al. A wearable hand rehabilitation system with soft gloves. IEEE Trans Ind Inform 2021; 17(2): 943–952.
119. Li M, Wang T, Zhou Y, et al. A soft robotic glove for hand rehabilitation training controlled by movements of the healthy hand. In: 2020 17th International Conference on Ubiquitous Robots (UR), Kyoto, Japan, 22–26 June 2020. New York: IEEE.
120. Moraru E, Onose G. Current issues and considerations about the central role of rehabilitation therapies in the functional recovery of neurological impairments after stroke in adults. J Med Life 2014; 7(3): 368–372.
121. Harvey RL. Improving poststroke recovery: neuroplasticity and task-oriented training. Curr Treat Options Cardiovasc Med 2009; 11(3): 251–259.
122. Hoang CL, Salle JY, Mandigout S, et al. Physical factors associated with fatigue after stroke: an exploratory study. Top Stroke Rehabil 2012; 19(5): 369–376.
123. Bates B, Choi JY, Duncan PW, et al.; US Department of Defense and Department of Veterans Affairs. Veterans affairs/department of defense clinical practice guideline for the management of adult stroke rehabilitation care: executive summary. Stroke 2005; 36(9): 2049–2056.
124. Merians AS, Jack D, Boian R, et al. Virtual reality–augmented rehabilitation for patients following stroke. Phys Ther Rehabil 2002; 82(9): 898–915.
125. Holden MK. Virtual environments for motor rehabilitation: review. Cyberpsychol Behav 2005; 8(3): 187–211.
126. Connelly L, Jia Y, Toro ML, et al. A pneumatic glove and immersive virtual reality environment for hand rehabilitative training after stroke. IEEE Trans Neural Syst Rehabil Eng 2010; 18(5): 551–559.
127. Merians AS, Fluet GG, Qiu Q, et al. Robotically facilitated virtual rehabilitation of arm transport integrated with finger movement in persons with hemiparesis. J Neuroeng Rehabil 2011; 8(1): 27–10.
128. Mitsopoulos K, Fiska V, Tagaras K, et al. Neurosuitup: system architecture and validation of a motor rehabilitation wearable robotics and serious game platform. Sensors 2023; 23(6): 3281.
[Abstract] Vagus Nerve Stimulation Paired With Rehabilitation for Post-Stroke Arm Impairment: One Year Follow-Up of the VNS-REHAB Pivotal Trial
Posted by Kostas Pantremenos in Neuroplasticity, Paretic Hand, REHABILITATION on February 19, 2024
Abstract
Background: Persistent post-stroke impairment of the arm and hand is debilitating after stroke. Pairing vagus nerve stimulation (VNS) with upper extremity (UE) rehabilitation improves such deficits after 5 months and was approved by the FDA in 2021. Here, we present 1-year outcomes from the VNS-REHAB pivotal trial.
Methods: Stroke participants with moderate-to-severe UE impairment were randomized to task-specific rehabilitation plus either active VNS (n=53, VNS) or sham VNS (n=55, Control). After baseline assessment (Pre-therapy), both groups did 6 wk. of in-clinic therapy followed by a 3-mo. home exercise program combined with active or sham VNS (post90). Controls then crossed over to receive 6 wk. of active VNS followed by a 3-mo. home exercise program (Cross-over post90). Both groups continued active VNS with a home exercise program through 1 year, after which change from Pre-therapy baseline in Fugl-Meyer Assessment-Upper Extremity (FMA-UE) and Wolf Motor Function Test (WMFT) scores were obtained. To determine whether participants made additional gains, 1 year outcome scores (n=70) were also compared to post90 (n=38, VNS) and Cross-over post90 (n=32, Control) scores. Data was available from 74 participants at one year, with others not available mainly due to COVID-19.
Results: At 1-year, both FMA-UE and WMFT scores improved from Pre-therapy baseline by 5.3±6.9 (CI=3.7-6.9, p<0.001) and 0.51±0.52 (CI=0.39-0.63, p<0.001) points, respectively. FMA-UE change at 1-year was not significantly different from the post90 (VNS) and Cross-over post90 (control) timepoints (n=70, mean difference: -0.3±4.1, CI=-1.3-0.67, p=0.52), but WMFT was, by an additional 0.09 points (n=70, mean difference: 0.09±0.35, CI=0.01-0.18, p=0.03), indicating that participants either improved or maintained motor gains through 1 year.
Conclusion: Improvements in arm and hand function with VNS were maintained at 1-year follow-up, supporting use of VNS paired with rehabilitation as a long-term treatment option for individuals with post-stroke UE impairment. Limitations include sample size and lack of details of therapeutic regimens over the long term. Future studies and an ongoing clinical registry will explore the long-term impact of active VNS in real-world settings.
[ARTICLE] Stroke patients’ motivation for home-based upper extremity rehabilitation with eHealth tools – Full Text
Posted by Kostas Pantremenos in Paretic Hand, REHABILITATION, Tele/Home Rehabilitation on February 18, 2024
Abstract
Purpose
eHealth-based exercise therapies were developed to increase stroke patients’ adherence to home-based motor rehabilitation. However, these eHealth tools face a rapid decrease in use after a couple of weeks. This study investigates stroke patients’ motivation for home-based upper extremity rehabilitation with eHealth tools and their relation with Basic Psychological Needs.
Materials and methods
This is a qualitative study using thematic analysis. We conducted semi-structured interviews with stroke patients with upper extremity motor impairments, who were discharged home from a rehabilitation centre, after they interacted with a novel eHealth coach demonstrator in their homes for five consecutive days.
Results
We included ten stroke patients. Thematic analysis resulted in eight themes for home-based rehabilitation motivation: Curiosity, Rationale, Choice, Optimal challenge, Reference, Encouragement, Social Support and Trustworthiness. Those themes are embedded into three Basic Psychological Needs: “Autonomy”, “Competence”, and “Relatedness”.
Conclusion
Eight motivational themes related to the three Basic Psychological Needs describe stroke patients’ motivation for home-based upper extremity rehabilitation. We recommend considering those themes when developing a home-based eHealth intervention for stroke patients to increase the alignment of eHealth tools to the patient’s needs and reduce motivational decreases in home-based rehabilitation.
Implications for rehabilitation
- Stroke patients show motivational decreases and decreased use of eHealth tools in home-based rehabilitation after a couple of weeks.
- Eight motivational themes describe home-based rehabilitation motivation in stroke patients: Curiosity, Rationale, Choice, Optimal challenge, Reference, Encouragement, Social Support and Trustworthiness.
- Those themes are embedded into three Basic Psychological Needs: “Autonomy”, “Competence”, and “Relatedness”.
- Those themes should be considered when developing a home-based eHealth intervention for stroke patients to increase the alignment of eHealth tools to the patient’s needs and reduce motivational decreases in home-based rehabilitation.
Introduction
Over two-thirds of stroke survivors experience upper extremity (UE) motor impairment, limiting capacity and resulting in significant daily functioning limitations and decreased quality of life [Citation1]. Rehabilitation programs involve stimulation of UE use and intensive UE exercises (e.g. a high number of repetitions per time unit) [Citation2]. Adherence to intensive daily UE use and exercise is essential to optimise UE capacity and daily life functioning [Citation3–5]. Exercise adherence may be specifically challenging for patients discharged from the controlled and supportive environment of a rehabilitation facility and expected to continue exercising at home [Citation6–8].
One of the most common perceived barriers to adherence to rehabilitation exercises and physical activity in stroke patients is a lack of motivation [Citation9]. Motivation can be generally explained as a fundamental “drive” towards change. According to Deci and Ryan’s Self Determination Theory (SDT), motivation has three underlying Basic Psychosocial Needs (BPNs): Autonomy, Competence and Relatedness (10). The need for autonomy refers to the belief in being agentic and volitional in your actions, and it implies self-initiation and a choice over your behaviour. The need for competence refers to feeling capable and effective in the physical and social environment. The need for relatedness refers to connecting to a community or group and having a secure relational base [Citation10]. These three BPNs are critical resources for psychological well-being and autonomous, self-determined motivation and satisfying them increases motivation to pursue desired behaviour [Citation10]. Literature shows that higher motivation levels positively correlate with higher adherence to a rehabilitation program [Citation11,Citation12] and better rehabilitation outcomes [Citation3,Citation4,Citation13].
Various motivational strategies targeting at least one of the three BPNs are used in face-to-face sessions between therapists and patients [Citation14,Citation15]. Examples of this are the application of patients’ preferences (increasing autonomy), control of task difficulty (increasing competence) and involvement of a family member (increasing relatedness) [Citation14,Citation15]. During home-based rehabilitation, patients’ motivational needs are expected to differ from inpatient rehabilitation since there is no direct supervision, less face-to-face contact between patients and therapists and more distraction from the physical and social environment. Besides, stroke often affects the social relationships and roles of the patient in their home environment, for example, family members may become informal caregivers, and patients are less involved in community activities [Citation16,Citation17].
Recently, a Delphi study was conducted to identify strategies to facilitate adherence to home-based exercises after a stroke [Citation18]. Multiple strategies were classified, including using eHealth applications to prescribe exercises, provide feedback and motivate patients to follow home-based rehabilitation. However, the expert panel in this study did not include stroke survivors, and the study does not provide insights into patients’ needs [Citation18]. Since many eHealth applications face a rapid decrease in use and low adherence in the home environment [Citation19], a better understanding of their motivational needs regarding using home-based rehabilitation eHealth tools is needed.
To increase the understanding of motivation in stroke patients, evaluating the BPNs in combination with patient-specific contextual and personal factors that influence motivation is important. Multiple studies documented motivation as the interplay of individual characteristics, including individual factors such as personality, psychological attributes and health status, and contextual factors, which are the social context and the culturally enforced norms [Citation11,Citation14,Citation20,Citation21]. BPNs, as described by Deci and Ryan, are generic and do not specifically take into account these personal and contextual factors. Moreover, the feeling of autonomy, competence, and social roles and relationships change due to a chronic disabling condition, such as stroke [Citation22]. Thus, a specific patient population’s characteristics and home environment should not be neglected when providing or developing home-based rehabilitation.
This study investigates stroke patients’ motivation for home-based UE rehabilitation using eHealth tools and how this is embedded in the three BPNs. To account for the specific personal and contextual factors, we choose a qualitative study design, allowing us to acquire rich personal and contextual data conducted within a representative group of stroke patients at their own homes. With this knowledge, we expect to better align eHealth tools to the patient’s needs, resulting in increased therapy adherence. […]

Figure 1. The interactive demonstrator “EDO” and the three exercises included in the interface: (a) cylinder grasp, (b) pinch grasp, and (c) lifting and tilting movement.
A three-panel figure shows a tablet, a cylindrical-shaped object, and the arm and hand of a person performing three different exercises. In panel a), the person’s hand holds the object with a cylindrical grasp, and the object turns orange. Panel b) shows the person’s hand performing a pinch grip on top of the cylindrical object while the object turns green. In panel c), the person’s hand holds the object in a horizontal position without ground support, while the object turns green.
[Abstract] Effects of Circuit Class Training Versus Individual, Task Specific Training on Upper Extremity Function in Chronic Stroke Patients
Posted by Kostas Pantremenos in Paretic Hand, Spasticity on February 15, 2024
Abstract

Background: Stroke is a leading contributor to disability globally, emphasizing the need for effective rehabilitation techniques. Circuit class training (CCT) and individual, task-specific training (ITST) have emerged as potential approaches for enhancing upper extremity function in stroke survivors. Comparative analyses of their efficacy, especially among chronic stroke patients, are scant.
Objective: This study aimed to evaluate and compare the impacts of CCT and ITST on upper extremity spasticity, motor function, and quality of life in individuals with chronic stroke.
Methods: In a randomized controlled trial, 36 chronic stroke patients were allocated to either CCT or ITST groups. Participants were aged 45-70 years, had experienced a single stroke episode, and were at least 6 months post-stroke, with specific inclusion criteria regarding spasticity and motor function levels. The interventions were delivered for 1.5 hours daily, five days a week, over eight weeks. Outcomes were measured using the Modified Ashworth Scale (MAS) for spasticity, Functional Independence Measure for Upper Extremity (FMA-UE) for motor function, and Stroke-Specific Quality of Life (SS-QOL) scale for quality of life, analyzed using SPSS version 25.
Results: Post-intervention, both CCT and ITST participants exhibited significant improvements in their outcomes. MAS scores showed a reduction in spasticity, with average improvements not significantly differing between the groups. FMA-UE scores increased by an average of 10 points in both groups, indicating enhanced motor function without a significant difference between the groups (p > 0.05). SS-QOL scores improved by an average of 20 points in each group, reflecting better quality of life, with no significant intergroup difference observed.
Conclusion: The study concludes that CCT and ITST are equally effective in ameliorating upper extremity spasticity, motor function, and quality of life among chronic stroke patients. The selection between CCT and ITST can thus be personalized based on patient preferences, available resources, and logistical considerations, maintaining rehabilitation efficacy.
References
Adeagbo CA, Olawale OA, Gbiri CAO. Transcranial direct current stimulation and repetitive functional task-oriented programme for upper limb functional rehabilitation in stroke survivors. Physical Therapy Reviews. 2021;26(6):420-7.
Alsubiheen AM, Choi W, Yu W, Lee H. The effect of task-oriented activities training on upper-limb function, daily activities, and quality of life in chronic stroke patients: A randomized controlled trial. International journal of environmental research and public health. 2022;19(21):14125.
Alwhaibi RM, Mahmoud NF, Zakaria HM, Badawy WM, Elzanaty MY, Ragab WM, et al. A comparative study on the effect of task specific training on right versus left chronic stroke patients. International journal of environmental research and public health. 2020;17(21):7950.
Anandan D, PK TN, Arun B, Priya V. Effect of task specific training with proprioceptive neuromuscular facilitation on stroke survivors. Biomedicine. 2020;40(3):363-6.
Bastola P, Singh P, Pinto D. A comparison of the effect of resistance training on upper extremity motor function, motor recovery, and quality of life in sub-acute stroke participants. Medical Journal of Dr DY Patil University. 2021;14(2):219-25.
Bovonsunthonchai S, Aung N, Hiengkaew V, Tretriluxana J. A randomized controlled trial of motor imagery combined with structured progressive circuit class therapy on gait in stroke survivors. Scientific Reports. 2020;10(1):6945.
da Silva ESM, Ocamoto GN, Santos-Maia GLd, de Fatima Carreira Moreira Padovez R, Trevisan C, de Noronha MA, et al. The effect of priming on outcomes of task-oriented training for the upper extremity in chronic stroke: a systematic review and meta-analysis. Neurorehabilitation and Neural Repair. 2020;34(6):479-504.
Deshpande S, Mohapatra S, Girish N. Influence of task-oriented circuit training on upper limb function among rural community-dwelling survivors of stroke. International Journal of Therapy And Rehabilitation. 2020;27(8):1-8.
Doğan M, Ayvat E, Kılınç M. Telerehabilitation versus virtual reality supported task-oriented circuit therapy on upper limbs and trunk functions in patients with multiple sclerosis: A randomized controlled study. Multiple Sclerosis and Related Disorders. 2023;71:104558.
Donnellan-Fernandez K, Ioakim A, Hordacre B. Revisiting dose and intensity of training: Opportunities to enhance recovery following stroke. Journal of Stroke and Cerebrovascular Diseases. 2022;31(11):106789.
Dorsch S, Carling C, Cao Z, Fanayan E, Graham PL, McCluskey A, et al. Bobath therapy is inferior to task-specific training and not superior to other interventions in improving arm activity and arm strength outcomes after stroke: a systematic review. Journal of physiotherapy. 2023;69(1):15-22.
Eldemir S, Guclu-Gunduz A, Eldemir K, Saygili F, Yilmaz R, Akbostancı MC. The effect of task-oriented circuit training-based telerehabilitation on upper extremity motor functions in patients with Parkinson’s disease: A randomized controlled trial. Parkinsonism & Related Disorders. 2023;109:105334.
Friel KM, Ferre CL, Brandao M, Kuo H-C, Chin K, Hung Y-C, et al. Improvements in upper extremity function following intensive training are independent of corticospinal tract organization in children with unilateral spastic cerebral palsy: a clinical randomized trial. Frontiers in Neurology. 2021;12:660780.
Gnanaprakasam A, Karthikbabu S, Ravishankar N, Solomon JM. Effect of task-based bilateral arm training on upper limb recovery after stroke: A systematic review and meta-analysis. Journal of Stroke and Cerebrovascular Diseases. 2023;32(7):107131.
Hsu H-Y, Kuan T-S, Tsai C-L, Wu P-T, Kuo Y-L, Su F-C, et al. Effect of a novel perturbation-based pinch task training on sensorimotor performance of upper extremity for patients with chronic stroke: A pilot randomized controlled trial. Archives of Physical Medicine and Rehabilitation. 2021;102(5):811-8.
Ibrahim R, Lawal IU, Joseph C. Intensity of Task-Specific Training for Functional Ability Post-stroke: Protocol for a Systematic Review. 2021.
Johar MN, Nordin NAM, Yusoff YAM. Effects of Game-Based Task-Oriented Circuit Training on Physical Functions of Stroke Survivors: A Pilot Study in A State Hospital in Kelantan, Malaysia. Asian Journal of Medicine and Biomedicine. 2021;5(S2):5-14.
Kim S-M, Kang S-H. The Effects of Task-Oriented Circuit Training Using Unstable Surface on Balance, Walking and Balance Confidence in Subacute Stroke Patients. Journal of The Korean Society of Integrative Medicine. 2021;9(4):211-23.
Lima ACd, Christofoletti G. Exercises with action observation contribute to upper limb recovery in chronic stroke patients: a controlled clinical trial. Motriz: Revista de Educação Física. 2020;26.
Llorens R, Fuentes MA, Borrego A, Latorre J, Alcañiz M, Colomer C, et al. Effectiveness of a combined transcranial direct current stimulation and virtual reality-based intervention on upper limb function in chronic individuals post-stroke with persistent severe hemiparesis: A randomized controlled trial. Journal of neuroengineering and rehabilitation. 2021;18:1-13.
Lotter JK, Henderson CE, Plawecki A, Holthus ME, Lucas EH, Ardestani MM, et al. Task-specific versus impairment-based training on locomotor performance in individuals with chronic spinal cord injury: a randomized crossover study. Neurorehabilitation and neural repair. 2020;34(7):627-39.
Martins JC, Nadeau S, Aguiar LT, Scianni AA, Teixeira-Salmela LF, De Morais Faria CDC. Efficacy of task-specific circuit training on physical activity levels and mobility of stroke patients: A randomized controlled trial. NeuroRehabilitation. 2020;47(4):451-62.
Mattos DJ, Rutlin J, Hong X, Zinn K, Shimony JS, Carter AR. White matter integrity of contralesional and transcallosal tracts may predict response to upper limb task-specific training in chronic stroke. NeuroImage: Clinical. 2021;31:102710.
Mawase F, Cherry-Allen K, Xu J, Anaya M, Uehara S, Celnik P. Pushing the rehabilitation boundaries: hand motor impairment can be reduced in chronic stroke. Neurorehabilitation and neural repair. 2020;34(8):733-45.
McDonell I, Barr C, van den Berg M. Implementing circuit class training can increase therapy time and functional independence in people with stroke receiving inpatient rehabilitation: findings from a retrospective observational clinical audit. Physiotherapy Theory and Practice. 2023:1-7.
Mooney RA, Cirillo J, Stinear CM, Byblow WD. Neurophysiology of motor skill learning in chronic stroke. Clinical Neurophysiology. 2020;131(4):791-8.
Nath D, Singh N, Saini M, Banduni O, Kumar N, Srivastava MP, et al. Clinical potential and neuroplastic effect of targeted virtual reality based intervention for distal upper limb in post-stroke rehabilitation: a pilot observational study. Disability and Rehabilitation. 2023:1-10.
Palimeris S. Combining a Tailored Strength Training Program with Transcranial Direct-Current Stimulation (tDcs) to Improve Upper Extremity Function in Chronic Stroke Patients: McGill University (Canada); 2020.
Roos MA, Thielman GT, Packel L, Moelter ST, Khakhina S, Klase ZA. The Impact of a Functional Circuit Training Program in People with ChronicStroke: A Non-Randomized Feasibility Study. 2021.
Zaman T, Mukhtar T, Waseem Zaman M, Shahid MN, Bibi S, Fatima A. Effects of task-oriented training on dexterous movements of hands in post stroke patients. International Journal of Neuroscience. 2024;134(2):175-83.
[WEB] Enspire announces publication of trial evaluating DBS plus rehab for stroke patients
Posted by Kostas Pantremenos in Paretic Hand, REHABILITATION on February 12, 2024
Enspire DBS Therapy has announced the publication of the results of the EDEN trial—an early feasibility study led by Cleveland Clinic (Cleveland, USA) researchers assessing electrical stimulation of the dentate nucleus for upper-extremity hemiparesis due to ischaemic stroke—in Nature Medicine.
The EDEN study was designed to evaluate whether deep brain stimulation (DBS) plus rehabilitation therapy (DBS plus rehab) would improve motor function in post-ischaemic stroke patients more effectively than rehab alone.
The study demonstrated that the majority of participants (9/12) showed the most significant improvements in both motor impairment and function during the combination therapy, DBS plus rehab. Importantly, an Enspire press release notes, participants with at least minimal preservation of distal motor function at enrolment showed clinically significant gains. The safety profile of this therapy is similar to other approved DBS therapies, the release adds.
These findings build on more than a decade of preclinical work led by principal investigators Andre Machado and Kenneth Baker (both Cleveland Clinic, Cleveland, USA). Machado patented the DBS method in stroke recovery to which Enspire has an exclusive license. He also holds stock options and equity ownership rights with Enspire, and serves as the company’s chief scientific officer.
“We saw patients in the study regain levels of function and independence they did not have before enrolling in the research,” Machado said. “We look forward to expanding as we have begun the next phase of clinical investigation.”
Enspire has also launched RESTORE, a pivotal study continuing to investigate the safety and effectiveness of DBS plus rehab in patients with chronic upper-extremity impairment due to stroke. The study has received investigational device exemption (IDE) approval from the US Food and Drug Administration (FDA) and is actively recruiting patients.
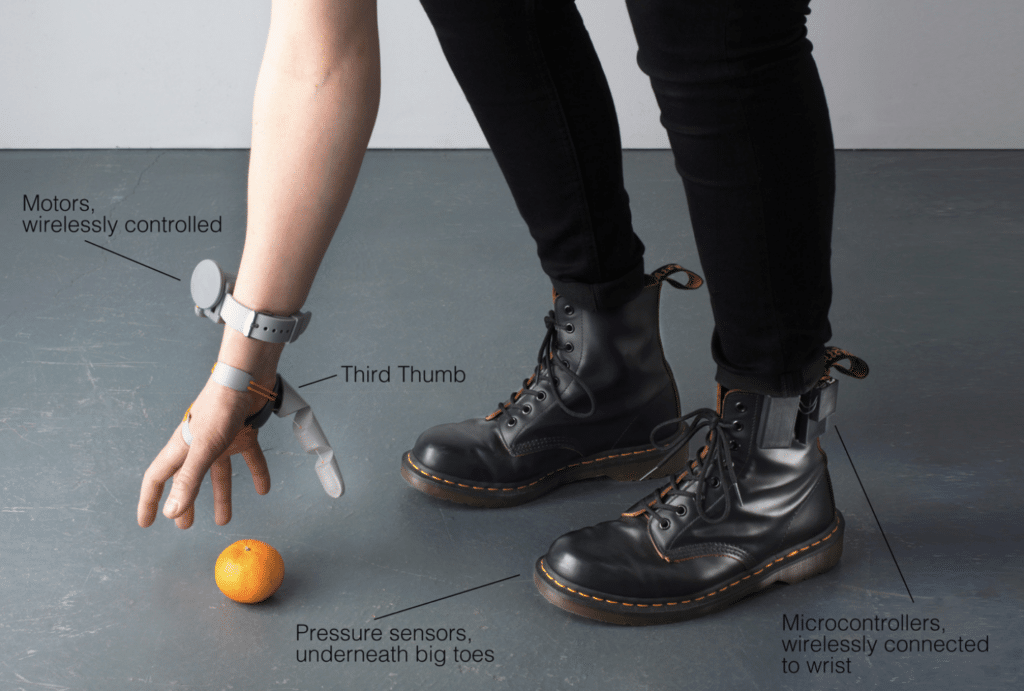
[WEB] This woman just created a robotic third thumb and it’s a total gamechanger
Posted by Kostas Pantremenos in Assistive Technology, Rehabilitation robotics on February 11, 2024

If you could have an extra body part, what would it be?
An augmentation designer has chosen a third thumb.
Sounds mad – and it looks it too – but it turns out an extra thumb would be pretty useful.
READ MORE! ‘World’s most advanced humanoid robot’ reveals what life will look like in 100 years
Designer Dani Clode developed a device called the ‘Third Thumb’ as part of a project at the Royal College of Art.
Clode wears the 3D-printed device on the side of the hand opposite her actual thumb near the pinky finger.
She then controls it with pressure sensors attached to her feet on the underside of the big toes.
Connected wirelessly, both toe sensors control different movements of the Third Thumb by immediately responding to subtle changes of pressure from the wearer.
To test the Third Thumb’s capability, a team from University College London were trained to use the device.
https://imasdk.googleapis.com/js/core/bridge3.619.0_en.html#goog_1293646367
417.3K
Water Firing Hypercar with NASA Technology | Hyperion
This involved focusing on tasks that helped increase the cooperation between their hand and the Third Thumb, such as picking up multiple balls or wine glasses with one hand.
They learned the basics of using the device very quickly, while the training enabled them to successfully improve their motor control, dexterity and hand-thumb coordination.
Participants were even able to use the device when distracted – building a wooden block tower while solving math problems – or while blindfolded.
So, it felt natural super quickly.


The project challenges the conventional idea of prosthetics by considering them as extensions rather than mere replacements.
A video posted to TikTok of the Third Thumb in action shows not everyone’s convinced, though.
“If we were meant to have six fingers, we would’ve been born with six fingers,” one user said.
“We’ve been fine without one for centuries. Why now?” another asked.
“People today don’t even know how to use their brain. What will they do with an extra finger?” another posted.
Some social media users suggested other body parts that would be more useful, like another pair or arms of even a tail.
Perhaps they’re projects for Clode to consider next.
[Abstract + References] Repetitive peripheral magnetic stimulation combined with transcranial magnetic stimulation in rehabilitation of upper extremity hemiparesis following stroke: a pilot study
Posted by Kostas Pantremenos in Paretic Hand, REHABILITATION on February 11, 2024
ABSTRACT
Objective: To investigate the effect of combined repetitive peripheral magnetic stimulation and transcranial magnetic stimulation on upper extremity function in subacute stroke patients.
Design: Pilot study.
Subjects: Subacute stroke patients.
Methods: Included patients were randomized into 3 groups: a central-associated peripheral stimulation (CPS) group, a central-stimulation-only (CS) group, and a control (C) group. The CPS group underwent a new paired associative stimulation (combined repetitive peripheral magnetic stimulation and transcranial magnetic stimulation), the CS group underwent repetitive transcranial magnetic stimulation, and the C group underwent sham stimulation. All 3 groups received physiotherapy after the stimulation or sham stimulation. The treatment comprised 20 once-daily sessions. Primary outcome was the Fugl-Meyer Assessment Upper Extremity (FMA-UE) score, and secondary outcomes were the Barthel Index and Comprehensive Functional Assessment scores, and neurophysiological assessments were mainly short-interval intracortical inhibition. A 3-group (CPS, CS, C) × 2-time (before, after intervention) repeated measures analysis of variance was conducted to determine whether changes in scores were significantly different between the 3 groups.
Results: A total of 45 patients were included in the analysis. Between-group comparisons on the FMA-UE demonstrated a significant improvement (group × time interaction, F2,42 = 4.86; p = 0.013; C vs CS, p = 0.020; C vs CPS, p = 0.016; CS vs CPS, p = 0.955). Correlation analysis did not find any substantial positive correlation between changes in FMA-UE and short-interval intracortical inhibition variables (C, r = –0.196, p = 0.483; CS, r = –0.169, p = 0.546; CPS, r = –0.424, p = 0.115).
Conclusion: This study suggests that the real-stimulus (CS and CPS) groups had better outcomes than the control (C) group. In addition, the CPS group showed a better trend in clinical and neurophysiological assessments compared with the CS group.
REFERENCES
Zhou M, Wang H, Zeng X, Yin P, Zhu J, Chen W, et al. Mortality, morbidity, and risk factors in China and its provinces, 1990–2017: a systematic analy-sis for the Global Burden of Disease Study 2017. Lancet 2019; 394: 1145–1158.
https://doi.org/10.1016/s0140-6736(19)30427-1 DOI: https://doi.org/10.1016/S0140-6736(19)30427-1
Kaji R. Global burden of neurological diseases highlights stroke. Nat Rev Neurol 2019; 15: 371–372.
https://doi.org/10.1038/s41582-019-0208-y DOI: https://doi.org/10.1038/s41582-019-0208-y
Coscia M, Wessel M, Chaudary U, Millán J, Micera S, Guggisberg A, et al. Neurotechnology-aided interventions for upper limb motor rehabilitation in severe chronic stroke. Brain 2019; 142: 2182–2197.
https://doi.org/10.1093/brain/awz181 DOI: https://doi.org/10.1093/brain/awz181
Wolf S, Holm S, Ingwersen T, Bartling C, Bender G, Birke G, et al. Pre-stroke socioeconomic status predicts upper limb motor recovery after inpatient neurorehabilitation. Ann Med 2022; 54: 1265–1276.
https://doi.org/10.1080/07853890.2022.2059557 DOI: https://doi.org/10.1080/07853890.2022.2059557
Leocani L, Cohen L, Wassermann E, Ikoma K, Hallett M. Human corticospinal excitability evaluated with transcranial magnetic stimulation during diffe-rent reaction time paradigms. Brain 2000;123: 1161–1173.
https://doi.org/10.1093/brain/123.6.1161 DOI: https://doi.org/10.1093/brain/123.6.1161
Koski L, Mernar T, Dobkin B. Immediate and long-term changes in corticomotor output in response to rehabilitation: correlation with functional impro-vements in chronic stroke. Neurorehabil Neural Repair 2004; 18: 230–249.
https://doi.org/10.1177/1545968304269210 DOI: https://doi.org/10.1177/1545968304269210
Kamo T, Wada Y, Okamura M, Sakai K, Momosaki R, Taito S. Repetitive peripheral magnetic stimulation for impairment and disability in people after stroke. Cochrane Database Syst Rev 2022; 9: CD011968.
https://doi.org/10.1002/14651858.CD011968.pub4 DOI: https://doi.org/10.1002/14651858.CD011968.pub4
Xiang H, Sun J, Tang X, Zeng K, Wu X. The effect and optimal parameters of repetitive transcranial magnetic stimulation on motor recovery in stroke patients: a systematic review and meta-analysis of randomized controlled trials. Clin Rehabil 2019; 33: 847–864.
https://doi.org/10.1177/0269215519829897 DOI: https://doi.org/10.1177/0269215519829897
Hummel F, Cohen L. Non-invasive brain stimulation: a new strategy to improve neurorehabilitation after stroke? Lancet Neurol2006; 5: 708–712.
https://doi.org/10.1016/s1474-4422(06)70525-7 DOI: https://doi.org/10.1016/S1474-4422(06)70525-7
Takeuchi N, Tada T, Toshima M, Matsuo Y, Ikoma K. Repetitive transcranial magnetic stimulation over bilateral hemispheres enhances motor function and training effect of paretic hand in patients after stroke. J Rehabil Med 2009; 41: 1049–1054.
https://doi.org/10.2340/16501977-0454 DOI: https://doi.org/10.2340/16501977-0454
Sakamoto D, Hamaguchi T, Murata K, Ito H, Nakayama Y, Abo M. Upper limb function recovery by combined repetitive transcranial magnetic stimulation and occupational therapy in patients with chronic stroke according to paralysis severity. Brain Sci 2023; 13: 284.
https://doi.org/10.3390/brainsci13020284 DOI: https://doi.org/10.3390/brainsci13020284
Juan Du, Yao W, Li J, Yang F, Hu J, Xu Q, et al. Motor network reorganization after repetitive transcranial magnetic stimulation in early stroke patients: a resting State fMRI study. Neurorehabil Neural Repair 2022; 36: 61–68.
https://doi.org/10.1177/15459683211054184 DOI: https://doi.org/10.1177/15459683211054184
Wang Q, Zhang D, Zhao Y, Hai H, Ma Y. Effects of high-frequency repetitive transcranial magnetic stimulation over the contralesional motor cortex on motor recovery in severe hemiplegic stroke: A randomized clinical trial. Brain Stimul 2020; 13: 979–986.
https://doi.org/10.1016/j.brs.2020.03.020 DOI: https://doi.org/10.1016/j.brs.2020.03.020
Wang R, Wang F, Huang S, Yang Y. High-frequency repetitive transcranial magnetic stimulation enhanced treadmill training effects on gait performance in individuals with chronic stroke: a double-blinded randomized controlled pilot trial. Gait Posture 2019; 68: 382–387.
https://doi.org/10.1016/j.gaitpost.2018.12.023 DOI: https://doi.org/10.1016/j.gaitpost.2018.12.023
Rosso C, Moulton E, Kemlin C, Leder S, Corvol J, Mehdi S, et al. Cerebello-motor paired associative stimulation and motor recovery in stroke: a rando-mized, sham-controlled, double-blind pilot trial. Neurotherapeutics 2022; 19: 491–500.
https://doi.org/10.1007/s13311-022-01205-y DOI: https://doi.org/10.1007/s13311-022-01205-y
Palmer J, Wolf S, Borich M. Paired associative stimulation modulates corticomotor excitability in chronic stroke: a preliminary investigation. Restor Neurol Neurosci 2018; 36: 183–194.
https://doi.org/10.3233/rnn-170785 DOI: https://doi.org/10.3233/RNN-170785
Silverstein J, Cortes M, Tsagaris K, Climent A, Gerber L, Oromendia C, et al. Paired associative stimulation as a tool to assess plasticity enhancers in chronic stroke. Front Neurosci 2019; 13: 792.
https://doi.org/10.3389/fnins.2019.00792 DOI: https://doi.org/10.3389/fnins.2019.00792
Tolmacheva A, Mäkelä J, Shulga A. Increasing the frequency of peripheral component in paired associative stimulation strengthens its efficacy. Sci Rep 2019; 9: 3849.
https://doi.org/10.1038/s41598-019-40474-0 DOI: https://doi.org/10.1038/s41598-019-40474-0
Yang T, Li X, Xia P, Wang X, Lu J, Wang L. Effects of rTMS combined with rPMS on stroke patients with arm paralysis after contralateral seventh cervical nerve transfer: a case-series. Int J Neurosci 2023; 133: 999–1007.
https://doi.org/10.1080/00207454.2022.2032044 DOI: https://doi.org/10.1080/00207454.2022.2032044
Sun T, Zhu G, Zheng Y, Mao Y, Hu Q, Song G, et al. Effects of paired associative magnetic stimulation between nerve root and cortex on motor function of lower limbs after spinal cord injury: study protocol for a randomized controlled trial. Neural Regen Res 2022; 17: 2459–2464.
https://doi.org/10.4103/1673-5374.339012 DOI: https://doi.org/10.4103/1673-5374.339012
Carson RG, Buick AR. Neuromuscular electrical stimulation-promoted plasticity of the human brain. J Physiol 2021; 599: 2375–2399.
https://doi.org/10.1113/JP278298 DOI: https://doi.org/10.1113/JP278298
Tarri M, Brihmat N, Gasq D, Lepage B, Loubinoux I, De BX, et al. Five-day course of paired associative stimulation fails to improve motor function in stroke patients. Ann Phys Rehabil Med 2018; 61: 78–84.
https://doi.org/10.1016/j.rehab.2017.11.002 DOI: https://doi.org/10.1016/j.rehab.2017.11.002
Kumru H, Albu S, Rothwell J, Leon D, Flores C, Opisso E, et al. Modulation of motor cortex excitability by paired peripheral and transcranial magnetic stimulation. Clin Neurophysiol 2017; 128: 2043–2047.
https://doi.org/10.1016/j.clinph.2017.06.041 DOI: https://doi.org/10.1016/j.clinph.2017.06.041
Liu Liping, Chen Weiqi, Zhou Hongyu, Duan Wanying, Li Shujuan, Huo Xiaochuan, et al. Chinese Stroke Association guidelines for clinical management of cerebrovascular disorders: executive summary and 2019 update of clinical management of ischaemic cerebrovascular diseases. Stroke Vasc Neurol 2020; 5(2): 159–176. DOI:10.1136/svn-2020-000378 DOI: https://doi.org/10.1136/svn-2020-000378
Noh J, Lim J, Choi T, Jang S, Pyun S. Effects and safety of combined rTMS and action observation for recovery of function in the upper extremities in stroke patients: a randomized controlled trial. Restor Neurol Neurosci 2019; 37: 219–230.
https://doi.org/10.3233/rnn-180883 DOI: https://doi.org/10.3233/RNN-180883
Liz L, Silva TG, Michaelsen SM. Validity, reliability, and measurement error of the remote Fugl-Meyer assessment by videoconferencing: Tele-FMA. Phys Ther 2023; 103: pzad054.
https://doi.org/10.1093/ptj/pzad054 DOI: https://doi.org/10.1093/ptj/pzad054
Beaulieu L, Milot M. Changes in transcranial magnetic stimulation outcome measures in response to upper-limb physical training in stroke: a systematic review of randomized controlled trials. Ann Phys Rehabil Med 2018; 61: 224–234.
https://doi.org/10.1016/j.rehab.2017.04.003 DOI: https://doi.org/10.1016/j.rehab.2017.04.003
Scarpino M, Lanzo G, Salimova M, Lolli F, Del VA, Cossu C, et al. Efficacy of high-frequency (15Hz) repetitive transcranial magnetic stimulation (rTMS) of the left premotor cortex/dorsolateral prefrontal cortex in decreasing cocaine intake (the MagneTox study): a study protocol for a randomized pla-cebo-controlled pilot trial. Neurophysiol Clin 2019; 49: 1–9.
https://doi.org/10.1016/j.neucli.2018.10.002 DOI: https://doi.org/10.1016/j.neucli.2018.10.002
Asao A, Wada K, Nomura T, Shibuya K. Time course changes in corticospinal excitability during repetitive peripheral magnetic stimulation combined with motor imagery. Neurosci Lett 2022; 771: 136427.
https://doi.org/10.1016/j.neulet.2021.136427 DOI: https://doi.org/10.1016/j.neulet.2021.136427
Demirtas A, Alonso M, Shetty R, Ronen I, Pascual-Leone A, Fregni F. Long-term effects of contralesional rTMS in severe stroke: safety, cortical excita-bility, and relationship with transcallosal motor fibers. NeuroRehabilitation 2015; 36: 51–59.
https://doi.org/10.3233/nre-141191 DOI: https://doi.org/10.3233/NRE-141191
Emara T, Moustafa R, ElNahas N, ElGanzoury A, Abdo T, Mohamed S, et al. Repetitive transcranial magnetic stimulation at 1Hz and 5Hz produces sustained improvement in motor function and disability after ischaemic stroke. Eur J Neurol 2010; 17: 1203–1209.
https://doi.org/10.1111/j.1468-1331.2010.03000.x DOI: https://doi.org/10.1111/j.1468-1331.2010.03000.x
Di G, Pellegrino G, Assenza G, Capone F, Ferreri F, Formica D, et al. Modulation of brain plasticity in stroke: a novel model for neurorehabilitation. Nat Rev Neurol 2014; 10: 597–608.
https://doi.org/10.1038/nrneurol.2014.162 DOI: https://doi.org/10.1038/nrneurol.2014.162
Le Q, Qu Y, Tao Y, Zhu S. Effects of repetitive transcranial magnetic stimulation on hand function recovery and excitability of the motor cortex after stroke: a meta-analysis. Am J Phys Med Rehabil 2014; 93: 422–430.
https://doi.org/10.1097/phm.0000000000000027 DOI: https://doi.org/10.1097/PHM.0000000000000027
Du J, Tian L, Liu W, Hu J, Xu G, Ma M, et al. Effects of repetitive transcranial magnetic stimulation on motor recovery and motor cortex excitability in patients with stroke: a randomized controlled trial. Eur J Neurol 2016; 23: 1666–1672.
https://doi.org/10.1111/ene.13105 DOI: https://doi.org/10.1111/ene.13105
Khedr E, Etraby A, Hemeda M, Nasef A, Razek A. Long-term effect of repetitive transcranial magnetic stimulation on motor function recovery after acute ischemic stroke. Acta Neurol Scand 2010; 121: 30–37.
https://doi.org/10.1111/j.1600-0404.2009.01195.x DOI: https://doi.org/10.1111/j.1600-0404.2009.01195.x
Du J, Yang F, Hu J, Hu J, Xu Q, Cong N, et al. Effects of high- and low-frequency repetitive transcranial magnetic stimulation on motor recovery in early stroke patients: Evidence from a randomized controlled trial with clinical, neurophysiological and functional imaging assessments. Neuroimage Clin 2019; 21: 101620.
https://doi.org/10.1016/j.nicl.2018.101620 DOI: https://doi.org/10.1016/j.nicl.2018.101620
Kim WJ, Rosselin C, Amatya B, Hafezi P, Khan F. Repetitive transcranial magnetic stimulation for management of post-stroke impairments: An over-view of systematic reviews. J Rehabil Med 2020; 52.
https://doi.org/10.2340/16501977-2637. DOI: https://doi.org/10.2340/16501977-2637
Gao B, Sun C, Xia G, Zhou S, Zhang Y, Mao Y, et al. Paired associated magnetic stimulation promotes neural repair in the rat middle cerebral artery occlusion model of stroke. Neural Regen Res 2020; 15: 2047–2056.
https://doi.org/10.4103/1673-5374.282266 DOI: https://doi.org/10.4103/1673-5374.282266
Xing Y, Zhang Y, Li C, Luo L, Hua Y, Hu J, et al. Repetitive transcranial magnetic stimulation of the brain after ischemic stroke: mechanisms from animal models. Cell Mol Neurobiol 2023; 43: 1487–1497.
https://doi.org/10.1007/s10571-022-01264-x DOI: https://doi.org/10.1007/s10571-022-01264-x
Bates K, Rodger J. Repetitive transcranial magnetic stimulation for stroke rehabilitation-potential therapy or misplaced hope? Restor Neurol Neurosci 2015; 33: 557–569.
https://doi.org/10.3233/rnn-130359 DOI: https://doi.org/10.3233/RNN-130359
Momosaki R, Yamada N, Ota E, Abo M. Repetitive peripheral magnetic stimulation for activities of daily living and functional ability in people after stroke. Cochrane Database Syst Rev 2017; 6: CD011968.
https://doi.org/10.1002/14651858.CD011968.pub2 DOI: https://doi.org/10.1002/14651858.CD011968.pub2
Sui Y, Tong L, Zhang X, Song Z, Guo T. Effects of paired associated stimulation with different stimulation position on motor cortex excitability and upper limb motor function in patients with cerebral infarction. J Clin Neurosci 2021; 90: 363–369.
https://doi.org/10.1016/j.jocn.2021.06.028 DOI: https://doi.org/10.1016/j.jocn.2021.06.028
Premoli I, Király J, Müller F, Zipser C, Rossini P, Zrenner C, et al. Short-interval and long-interval intracortical inhibition of TMS-evoked EEG potentials. Brain Stimul 2018; 11: 818–827.
https://doi.org/10.1016/j.brs.2018.03.008 DOI: https://doi.org/10.1016/j.brs.2018.03.008
Mello E, Cohen L, Monteiro DAS, Conti J, Andrade K, Tovar MF, et al. Increase in short-interval intracortical facilitation of the motor cortex after low-frequency repetitive magnetic stimulation of the unaffected hemisphere in the subacute phase after stroke. Neural Plast 2015; 2015: 407320.
https://doi.org/10.1155/2015/407320 DOI: https://doi.org/10.1155/2015/407320
Ferreiro AK, Conforto A. Decreased short-interval intracortical inhibition correlates with better pinch strength in patients with stroke and good motor recovery. Brain Stimul 2018; 11: 772–774.
https://doi.org/10.1016/j.brs.2018.01.030 DOI: https://doi.org/10.1016/j.brs.2018.01.030
Harvey R, Edwards D, Dunning K, Fregni F, Stein J, Laine J, et al. Randomized sham-controlled trial of navi-gated repetitive transcranial magnetic stimulation for motor recovery in stroke. Stroke 2018; 49: 2138–2146.
https://doi.org/10.1161/strokeaha.117.020607 DOI: https://doi.org/10.1161/STROKEAHA.117.020607
