Posts Tagged Brain plasticity
[ARTICLE] Peak Activation Shifts in the Sensorimotor Cortex of Chronic Stroke Patients Following Robot-assisted Rehabilitation Therapy – Full Text
Posted by Kostas Pantremenos in Paretic Hand, Rehabilitation robotics on July 31, 2021
ABSTRACT
Background:
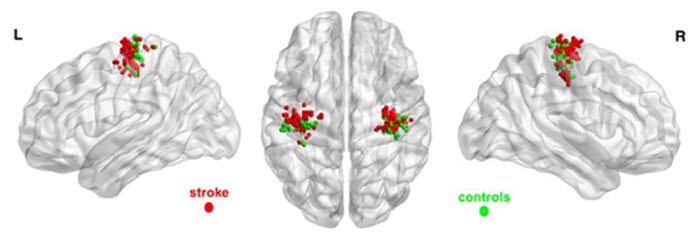
Ischemic stroke is the most common cause of complex chronic disability and the third leading cause of death worldwide. In recovering stroke patients, peak activation within the ipsilesional primary motor cortex (M1) during the performance of a simple motor task has been shown to exhibit an anterior shift in many studies and a posterior shift in other studies.
Objective:
We investigated this discrepancy in chronic stroke patients who completed a robot-assisted rehabilitation therapy program.
Methods:
Eight chronic stroke patients with an intact M1 and 13 Healthy Control (HC) volunteers underwent 300 functional magnetic resonance imaging (fMRI) scans while performing a grip task at different force levels with a robotic device. The patients were trained with the same robotic device over a 10-week intervention period and their progress was evaluated serially with the Fugl-Meyer and Modified Ashworth scales. Repeated measure analyses were used to assess group differences in locations of peak activity in the sensorimotor cortex (SM) and the relationship of such changes with scores on the Fugl-Meyer Upper Extremity (FM UE) scale.
Results:
Patients moving their stroke-affected hand had proportionally more peak activations in the primary motor area and fewer peak activations in the somatosensory cortex than the healthy controls (P=0.009). They also showed an anterior shift of peak activity on average of 5.3-mm (P<0.001). The shift correlated negatively with FM UE scores (P=0.002).
Conclusion:
A stroke rehabilitation grip task with a robotic device was confirmed to be feasible during fMRI scanning and thus amenable to be used to assess plastic changes in neurological motor activity. Location of peak activity in the SM is a promising clinical neuroimaging index for the evaluation and monitoring of chronic stroke patients.
[…]
[WEB] BGU’s Negev Lab is bringing stroke rehab into the future
Posted by Kostas Pantremenos in Artificial intelligence, Neuroplasticity on July 30, 2021
Stroke is tricky. While its effects are well known, the best course of rehabilitation to return to functionality is still very much a mystery. This rehab lab turns translational science for answers.
By EHUD ZION-WALDOKS

The return-to-work rate for people after suffering a stroke has not significantly changed since the 1970s,” says Dr. Simona Bar-Haim, founding director of the Negev Lab at the ADI Negev-Nahalat Eran Rehabilitation Village in southern Israel.
Bar-Haim is not your typical academic. She has one foot firmly in the field, and one foot firmly in the academy as a member of the department of physical therapy in the Faculty of Health Sciences at Ben-Gurion University of the Negev (BGU). She also founded a start-up based on chaos theory to help people walk after suffering a stroke.
“Many people do not recover enough to return to work or to their regular lives,” she says.
What is more, as the population ages and lives longer, there are more and more people suffering strokes and then recovering only partial functionality. In Israel, 17,000-20,000 people a year suffer a stroke.
Stroke is tricky. While its effects are well known, the best course of rehabilitation to return to functionality is still very much a mystery, to which Bar-Haim and Dr. Lior Shmuelof, also of BGU, have devoted themselves to help solve.
Driven by that impetus to help and by her out-of-the-box way of thinking, Bar-Haim recently set up a translational rehabilitation lab at the rehabilitation village. Translational science tries to find solutions for real-world problems. At ADI Negev, the problems arise from the patients themselves, their doctors and caregivers, and the solutions are tested in conjunction with the patients.
There are several theories why stroke recovery has not progressed since the 1970s and the Negev Lab has been working to test them.
“We believe there is a critical period of three to six months after the stroke where recovery is most achievable because of the brain’s plasticity during that time,” says Shmuelof of the Negev Lab, the department of brain and cognitive sciences and the Zlotowski Center for Neuroscience at BGU. “If an animal suffers a stroke, it recovers fully. Why do animals recover, while people do not? One possibility is that an animal is active from the moment it happens. Now, if a person suffers a stroke, they spend the first week to 10 days lying in bed in the hospital, and then they spend a couple of hours a day doing physical therapy that does not translate to the real world.
”To confirm these hypotheses, Shmuelof is partnering with the MRI Imaging Center at Soroka-University Medical Center and researchers Profs. Alon Friedman, Ilan Shelef, Anat Horev and Gal Ben-Arie, with the aim of identifying the neural components associated with brain plasticity after injury.
Shmuelof will then take what he learns from MRI imaging and bring it back to the Negev Lab.
ADI NEGEV-Nahalat Eran is a fully equipped facility in a village setting, with residential care for people with multiple disabilities and complex medical conditions, an intensive care hospital wing for babies and adults, a paramedical center, hydrotherapy pool, special education school, green care farm, and a therapeutic horse stable and petting zoo.
It is set in an ideal and idyllic location. Winding paths run alongside the residents’ cottages. A stable for therapeutic riding anchors one side, while the Negev Lab anchors the other. The atmosphere is calm, quiet, happy and optimistic – a far cry from rehabilitation wards in large hospitals.
The ADI Negev-Nahalat Eran Rehabilitation Village was named in memory of Eran Almog, the late son of Didi and Maj.-Gen. (ret.) Doron Almog.
Fueled by his love for Eran, who was born with severe autism and intellectual disabilities, Doron Almog guided the creation of a residential and rehabilitative complex in Israel’s south, which has since become a home and family for more than 150 children and young adults with severe disabilities and complex medical conditions and provides a host of rehabilitative solutions for individuals from all backgrounds and levels of need.
While care is important, the vision is to provide cutting-edge treatment as well. The first step was the creation of the Negev Lab. Not far in the future, the village will also boast a rehabilitation hospital, which will be the biggest in southern Israel.
“ADI Negev is the ideal place to see what happens when people spend many more hours rehabilitating,” says Shmuelof. “What if they spend three hours a day or five hours a day? Would they recover faster and better? These are the kinds of questions we ask ourselves and have the ability to answer because of this unique lab.
”Existing movement tracking methods are not advanced enough to meet Shmuelof’s needs. Therefore, to track patients’ motion over the course of the day, the Negev Lab is developing methods to track not just walking but also arm movements.
“One of the things we noticed is that arm motion might not be completely impaired, but weakness causes people to compensate in other ways rather than moving their arms to regain functionality,” says Shmuelof.
“Putting a research lab in a rehabilitation village makes a lot of sense,” says Bar-Haim. “There, we can go directly to the residents and ask them what their needs are. We can also test out our technologies, which we make sure are fun and pleasant, on the residents and get their feedback.”
THAT IMMEDIATE feedback appealed to Prof. Ilana Nisky of BGU’s department of biomedical engineering, who has joined Bar-Haim in developing a belt that helps stroke patients improve their walking. She is an expert in haptics, which is the body’s sense of touch. Their project is being funded by the Israel Innovation Authority.
“When you design medical devices, you need to think beyond engineering and understand how they [the patients] will be using the devices,” says Nisky.
“One of the most important elements in walking is being able to feel the ground and knowing where your legs are without looking at them. That ability, which we take for granted, can be damaged by a stroke. So, we are designing a belt that is worn on the person’s skin under their clothes that will massage the person’s waist and help them with their terra sense – the sense of where the ground is and where their legs are,” she explains.
“What is truly groundbreaking in our belt is that it does not need to measure where the legs are relative to the ground. Instead, we rely on an artificial intelligence algorithm that we train on many examples of past walking to guess where the ground and legs should be at a given moment in time, based on a very simple sensor that is placed on the belt and in the center of the body. This way the only thing the person after stroke will need is the belt itself.
”The researchers have already developed a prototype, but in the future each belt will be customized to the individual person’s needs.
“We hope they will use the belt, and that the information it provides to them will be helpful,” Nisky says. “We also hope that if they use it a lot, then perhaps they will eventually be able to walk on their own without it.
”To make sure that they will indeed wear it a lot rather than buy it just to have it collect dust in the closet, the team also works with Ofer Canfi, a designer who graduated from the Bezalel Academy of Arts and Design and the Royal Academy of Arts, London, to make the belt look and feel nice, using advanced manufacturing procedures and hi-tech fabrics.
Nisky notes that now is the ideal moment for such research. Haptic devices have become much more pleasant to use. “It’s like a massage. What’s not to like?” she says, while artificial intelligence has reached the point where it can be harnessed for purposes such as physical therapy.
A future entrepreneurial hub
Another important advantage offered by the Negev Lab is its multidisciplinary nature.“We have clinicians, clinician-researchers, engineers and programmers, all working together,” says Nisky.Bar-Haim also envisions the Negev Lab as an entrepreneurial hub, a space where technologies from around the world can be tested and receive feedback from the people who stand to benefit from them.In fact, this vision is already a reality. The Negev Lab collaborates with Swiss-based Mindmaze, which designs virtual reality and computer simulations. It sends its latest technologies to the lab, where Shmuelof puts them through their paces with patients.One such program lets the patient control a dolphin on a screen by raising or lowering their arms. It has been a big hit with patients.“Seeing the dolphin move in response to my arm movements shows me how much I have improved,” one says.“Using the vest gives me hope that I will return to moving my hand easily,” another says. “At the end of a treatment session, I feel like my whole body got into it,” says a third.“People instinctively understand how to control the dolphin, and they enjoy it,” explains Shmuelof.“The simulation makes me feel like I’m playing a computer game at home, and I just want to pass level after level,” says a patient.That is encouraging feedback, because the Negev Lab wants to develop programs that people will actually use.
The rehabilitation hospital
In a welcome development, the ADI Negev-Nahalat Eran Neuro-Orthopedic Rehabilitative Hospital is nearing completion. Thanks to the support of multiple government ministries, JNF-USA and international donors, the hospital is set for completion late this year.It will have more than 100 beds and will provide unique research opportunities.“It will also be a unique research hospital, with an ethical and information technology infrastructure that will allow us to study most of the activities that will be carried out there,” says Bar-Haim.“In other words, a large proportion of what goes on in the hospital will be able to be researched and incorporated into academic studies. That is not the case in most hospitals around the world currently, not even in teaching hospitals.“Once the hospital is completed, the Negev Lab will move into its new space and become the largest and most advanced lab of its kind in Israel.” Bar-Haim and Shmuelof are excited about the opportunities to advance their understanding of how to rehabilitate stroke patients using the knowledge they will gain from researching at the hospital.“How active was the patient during the day? How well did she sleep? How long did she sit for? Measuring patients’ activity during the day will allow us to better understand how it affects their recovery, and to find ways to increase their activity during rehabilitation,” explains Shmuelof.“We are already receiving inquiries from around the world about this new lab,” he adds.
The future
While the South has lagged behind the Center in terms of medical and rehabilitative care, Bar-Haim and Doron Almog’s vision does not stop at achieving parity with the Center, but, rather, aims to exceed it.“We believe that residents of the South deserve the same quality of care as those in every other part of the country, and we believe that we can set the bar higher for rehabilitative care,” says Almog.“This village was founded on the principle that a person is a person no matter what, and this hospital and research lab are finally starting to realize our full vision. In this place, all people will be provided with the best possible care and loved beyond measure.“In the age of corona, the importance of the Negev Lab is clearer than ever before. Each and every day, our rehabilitation professionals empower people of all ages, backgrounds, and levels of need, giving them a new lease on life and returning them to their families in good health and renewed spirit.“But we can expedite this process and make it even more powerful through collaborative translational research. There are so many people hurting right now, and the groundbreaking research being done at the Negev Lab can change the face of rehabilitative care across Israel and around the world.”“I envision the Negev Lab and the ADI Negev-Nahalat Eran Neuro-Orthopedic Rehabilitative Hospital as the core of the future National Rehabilitative Institute of Israel,” declares Bar-Haim.
The writer is deputy spokesperson, international media at Ben-Gurion University.
[WEB] New glial cells discovered in the brain: Implications for brain repair
Posted by Kostas Pantremenos in Neuroplasticity on July 4, 2021
Summary: Neurons, nerve cells in the brain, are central players in brain function. However, a key role for glia, long considered support cells, is emerging. A research group has now discovered two new types of glial cells in the brain, by unleashing adult stem cells from their quiescent state. These new types of glia may play an important role in brain plasticity and repair.
FULL STORY
Neurons, nerve cells in the brain, are central players in brain function. However, a key role for glia, long considered support cells, is emerging. A research group at the University of Basel has now discovered two new types of glial cells in the brain, by unleashing adult stem cells from their quiescent state. These new types of glia may play an important role in brain plasticity and repair.
The brain is malleable well into adulthood. Brain plasticity is not only due to the formation of new nerve connections. Stem cells present in the adult brain also generate new nerve cells. For more than a hundred years, scientists have concentrated on investigating different types of nerve cells.
In the brain, however, another class of cells, called glia, are also essential for brain function. However, the importance of glial cells has been underestimated for decades. How many types of glia there are, how they develop and what roles they play are all still largely unexplored.
Stem cells — unleashed from quiescence
The research group of Prof. Fiona Doetsch at the Biozentrum of the University of Basel is investigating stem cells in the ventricular-subventricular zone in the adult mouse brain. In this region, many of the stem cells are in a quiescent state, sensing signals in the environment that stimulate them to awaken and transform into new nerve cells.
In their study in the journal Science, Doetsch’s team identified a molecular signal that awakened the stem cells from their quiescent state, allowing them to uncover multiple domains that give rise to glial cells in this stem cell reservoir.
Stem cells — birthplace of glial cells
“We found an activation switch for quiescent stem cells,” Doetsch explains. “It is a receptor that maintains the stem cells in their resting state. We were able to turn off this switch and thus activate the stem cells,” Doetsch says. In addition, the researchers were able to visualize the development of the stem cells into different glial cells in specific areas of the stem cell niche.
“Some of the stem cells did not develop into neurons, but into two different novel types of glial cells,” Doetsch reports. This brain region studied is therefore a birthplace for different types of glial cells as well as its role as a breeding ground for neurons.
“What was very unexpected was that one glial cell type was found attached to the surface of the wall of the brain ventricle, rather than in the brain tissue.” These cells are continuously bathed by cerebrospinal fluid and interact with axons from other brain areas, and therefore are poised to sense and integrate multiple long-range signals.
Glial cells — active in health and disease
The research team also found that both glial cell types were activated in a model of demyelination. These new glial cell types may therefore be a source of cells for repair in neurodegenerative diseases, such as multiple sclerosis or after injury.
As a next step, Doetsch would like to specifically trace these new glial cell types and to investigate their roles in normal brain function and how they respond in different physiological contexts. This will provide important clues to understanding brain plasticity and how the renewal and repair of neural tissue occurs.
Story Source:
Materials provided by University of Basel. Note: Content may be edited for style and length.
Journal Reference:
- Ana C. Delgado, Angel R. Maldonado-Soto, Violeta Silva-Vargas, Dogukan Mizrak, Thomas von Känel, Kelly R. Tan, Alex Paul, Aviv Madar, Henar Cuervo, Jan Kitajewski, Chyuan-Sheng Lin, Fiona Doetsch. Release of stem cells from quiescence reveals gliogenic domains in the adult mouse brain. Science, 2021; 372 (6547): 1205 DOI: 10.1126/science.abg8467
[WEB PAGE] The 2-Week Peak: Stroke Recovery
Posted by Kostas Pantremenos in Neuroplasticity, Recovery Plateau, REHABILITATION on February 24, 2021

The capacity of the human brain to recover and rewire itself peaks around 2 weeks after a stroke and diminishes over time, according to researchers who followed the recovery of 60 stroke patients up to 1 year after their stroke.
The study, conducted by researchers from London and Adelaide, was published in Neurorehabilitation and Neural Repair.
The multi-site study showed conclusive evidence that the brain only has a small window of opportunity to more easily repair itself after stroke, according to lead author Dr Brenton Hordacre, from the University of South Australia, a media release from the university explains.
“Earlier animal studies suggested this was the case, but this is the first time we have conclusively demonstrated this phenomenon exists in humans.”
— Dr Benton Hordacre
SCANNED BRAINS THROUGHOUT RECOVERY
The researchers scanned the brains of stroke survivors as they recovered over 12 months. They found that in the initial days following an ischemic stroke (caused by a blocked artery to the brain), the brain has a greater capacity to modify its neural connections and its plasticity is increased.
“It is during this early period after stroke that any physiotherapy is going to be most effective because the brain is more responsive to treatment. Earlier experiments with rats showed that within five days of an ischemic stroke they were able to repair damaged limbs and neural connections more easily than if therapy was delayed until 30 days post stroke,” they write.
The researchers used continuous transcranial magnetic stimulation (cTBS) to repetitively activate different hemispheres of the motor cortex to measure brain plasticity.
The Adelaide laboratory tested the stroke-damaged motor cortex, which is the main area that controls movement. The London laboratory tested the non-stroke damaged hemisphere which is also important to help recovery, the release continues.
“Our assessments showed that plasticity was strongest around two weeks after stroke in the non-damaged motor cortex. Contrary to what we expected, there was no change in the damaged hemisphere in response to cTBS.”
— Dr Hordacre, who adds that the findings confirm the importance of initiating therapy as soon as possible after a stroke
MORE THERAPY NEEDED FOR UPPER LIMB RECOVERY
Current evidence indicates that less than 8 minutes of daily therapy is dedicated to upper limb recovery within the first 4 weeks of a stroke.
“Delivering more treatment within this brief window is needed to help people recover after stroke. The next step is to identify techniques which prolong or even re-open a period of increased brain plasticity, so we can maximize recovery,” Hordacre concludes.
[Abstract] Neural plasticity: The substratum of music-based interventions in neurorehabilitation
Posted by Kostas Pantremenos in Music/Music therapy, Neuroplasticity on February 14, 2021
Abstract
Background: The plastic nature of the human brain lends itself to experience and training-based structural changes leading to functional recovery. Music, with its multimodal activation of the brain, serves as a useful model for neurorehabilitation through neuroplastic changes in dysfunctional or impaired networks. Neurologic Music Therapy (NMT) contributes to the field of neurorehabilitation using this rationale.
Objective: The purpose of this article is to present a discourse on the concept of neuroplasticity and music-based neuroplasticity through the techniques of NMT in the domain of neurological rehabilitation.
Methods: The article draws on observations and findings made by researchers in the areas of neuroplasticity, music-based neuroplastic changes, NMT in neurological disorders and the implication of further research in this field.
Results: A commentary on previous research reveal that interventions based on the NMT paradigm have been successfully used to train neural networks using music-based tasks and paradigms which have been explained to have cross-modal effects on sensorimotor, language and cognitive and affective functions.
Conclusions: Multimodal gains using music-based interventions highlight the brain plasticity inducing function of music. Individual differences do play a predictive role in neurological gains associated with such interventions. This area deserves further exploration and application-based studies.
[BLOG POST] Ten Fundamentals Of Rewiring Your Brain
Posted by Kostas Pantremenos in Neuroplasticity on October 22, 2020
Neuroplasticity has become a buzzword in psychology and scientific circles, as well as outside of them, promising that you can “rewire” your brain to improve everything from health and mental well-being to quality of life. There’s a lot of conflicting, misleading, and erroneous information out there.
So, exactly how does it work?
What Is Neuroplasticity
Just in case you’ve managed to miss all the hype, neuroplasticity is an umbrella term referring to the ability of your brain to reorganize itself, both physically and functionally, throughout your life due to your environment, behavior, thinking, and emotions. The concept of neuroplasticity is not new and mentions of a malleable brain go all of the way back to the 1800s, but with the relatively recent capability to visually “see” into the brain allowed by functional magnetic resonance imaging (fMRI), science has confirmed this incredible morphing ability of the brain beyond a doubt.
The concept of a changing brain has replaced the formerly held belief that the adult brain was pretty much a physiologically static organ or hard-wired, after critical developmental periods in childhood. While it’s true that your brain is much more plastic during the early years and capacity declines with age, plasticity happens all throughout your life.
For a thorough explanation of how plasticity physically happens in your brain, see blog: Masterpiece Or Mess.
How Neuroplasticity Shows Up In Your Life
Neuroplasticity makes your brain extremely resilient and is the process by which all permanent learning takes place in your brain, such as playing a musical instrument or mastering a different language. Neuroplasticity also enables people to recover from stroke, injury, and birth abnormalities, improve symptoms of autism, ADD and ADHD, learning disabilities and other brain deficits, pull out of depression and addictions, and reverse obsessive-compulsive patterns. (Read more: You’re Not Stuck With The Brain You’re Born With)
Neuroplasticity has far-reaching implications and possibilities for almost every aspect of human life and culture from education to medicine. Its limits are not yet known. However, this same characteristic, which makes your brain amazingly resilient, also makes it vulnerable to outside and internal, usually unconscious, influences. In his book The Brain That Changes Itself: Stories of Personal Triumph from the Frontiers of Brain Science, Norman Doidge calls this the “plastic paradox.” (Read more: Your Plastic Brain: The Good, The Bad, and The Ugly)
I know the power of neuroplasticity first hand, as I devised and performed my own homegrown, experience-dependent neuroplasticity-based exercises for years to recover from a brain injury, the result of a suicide attempt. Additionally, through extensive cognitive behavioral therapy, meditation, and mindfulness practices, all of which encourage neuroplastic change, I overcame depression, anxiety, and totally revamped my mental health and life.
But it was because of neuroplastic change that I became entrenched in depressive, anxious, obsessive, and over-reactive patterns in the first place.
Ten Fundamentals Of Neuroplasticity
Science has confirmed that you CAN access neuroplasticity for positive change in your own life in many ways, but it’s not quite as easy as some of the neuro-hype would have you believe. In the article, Neuroplasticity: can you rewire your brain?, Dr. Sarah McKay, neuroscientist, says:
Plasticity dials back ‘ON’ in adulthood when specific conditions that enable or trigger plasticity are met. ‘What recent research has shown is that under the right circumstances, the power of brain plasticity can help adults minds grow. Although certain brain machinery tends to decline with age, there are steps people can take to tap into plasticity and reinvigorate that machinery,’ explains Merzenich. These circumstances include focused attention, determination, hard work and maintaining overall brain health.
In his book, Soft-Wired: How the New Science of Brain Plasticity Can Change Your Life, Dr. Michael Merzenich (which Dr. McKay cites above), a leading pioneer in brain plasticity research and co-founder of Posit Science, lists ten core principles necessary for the remodeling of your brain to take place:
1. Change is mostly limited to those situations in which the brain is in the mood for it.
If you are alert, on the ball, engaged, motivated, ready for action, the brain releases the neurochemicals necessary to enable brain change. When disengaged, inattentive, distracted, or doing something without thinking that requires no real effort, your neuroplastic switches are “off.”
2. The harder you try, the more you’re motivated, the more alert you are, and the better (or worse) the potential outcome, the bigger the brain change.
If you’re intensely focused on the task and really trying to master something for an important reason, the change experienced will be greater.
3. What actually changes in the brain are the strengths of the connections of neurons that are engaged together, moment by moment, in time.
The more something is practiced, the more connections are changed and made to include all elements of the experience (sensory info, movement, cognitive patterns). You can think of it like a “master controller” being formed for that particular behavior which allows it to be performed with remarkable facility and reliability over time.
4. Learning-driven changes in connections increase cell-to-cell cooperation which is crucial for increasing reliability.
Merzenich explains this by asking you to imagine the sound of a football stadium full of fans all clapping at random versus the same people clapping in unison. He explains, “The more powerfully coordinated your [nerve cell] teams are, the more powerful and more reliable their behavioral productions.”
5. The brain also strengthens its connections between teams of neurons representing separate moments of successive things that reliably occur in serial time.
This allows your brain to predict what happens next and have a continuous “associative flow.” Without this ability, your stream of consciousness would be reduced to “a series of separate, stagnating puddles,” explains Merzenich.
6. Initial changes are temporary.
Your brain first records the change, then determines whether it should make the change permanent or not. It only becomes permanent if your brain judges the experience to be fascinating or novel enough or if the behavioral outcome is important, good or bad.
7. The brain is changed by internal mental rehearsal in the same ways and involving precisely the same processes that control changes achieved through interactions with the external world.
According to Merzenich, “You don’t have to move an inch to drive positive plastic change in your brain. Your internal representations of things recalled from memory work just fine for progressive brain plasticity-based learning.” See blog: Two Primary Ways to Drive Brain Neuroplasticity.
8. Memory guides and controls most learning.
As you learn a new skill, your brain takes note of and remembers the good attempts, while discarding the not-so-good trys. Then, it recalls the last good pass, makes incremental adjustments, and progressively improves.
9. Every movement of learning provides a moment of opportunity for the brain to stabilize – and reduce the disruptive power of – potentially interfering backgrounds or “noise.”
Each time your brain strengthens a connection to advance your mastery of a skill, it also weakens other connections of neurons that weren’t used at that precise moment. This negative plastic brain change erases some of the irrelevant or interfering activity in the brain.
10. Brain plasticity is a two-way street; it is just as easy to generate negative changes as it is positive ones.
You have a “use it or lose it” brain. It’s almost as easy to drive changes that impair memory and physical and mental abilities as it is to improve these things. Merzenich says that older people are absolute masters at encouraging plastic brain change in the wrong direction. See blog: Are You Unknowingly Contributing To Your Brain’s Decline?
[WEB SITE] Study Links Obesity to Brain Plasticity and Stroke, TBI Recovery
Posted by Kostas Pantremenos in Neuroplasticity, TBI on September 26, 2020
Posted by Deborah Overman | Sep 24, 2020

Severely overweight people are less likely to be able to re-wire their brains and find new neural pathways, a discovery that could have significant implications for people recovering from a stroke or brain injury.
In a new study published in Brain Sciences, researchers from UniSA and Deakin University show that brain plasticity is impaired in obese people, making it less likely that they can learn new tasks or remember things.
Using a series of experiments involving transcranial magnetic stimulation, the researchers tested 15 obese people aged between 18 and 60, and compared them with 15 people in a healthy-weight control group.
NORMAL BRAIN PLASTICITY IN HEALTHY-WEIGHT PEOPLE
Repeated pulses of electrical stimulation were applied to the brain to see how strongly it responded. The healthy-weight control group recorded significant neural activity in response to the stimulation, suggesting a normal brain plasticity response. In contrast, the response in the obese group was minimal, suggesting its capacity to change was impaired.
The findings provide the first physiological evidence of a link between obesity and reduced brain plasticity, UniSA researcher Dr Brenton Hordacre suggests.
Obesity is based on body mass index (BMI) which calculates the ratio between height and weight to determine body fat. An adult who has a BMI between 25 and 29.9 is considered overweight. Anything above that is obese.
“Obesity is already associated with a raft of adverse health effects, including a higher risk of cardiovascular disease, metabolic disorders and dementia. For the first time, we found that obesity was associated with impaired brain function, adding further support for the need to address the obesity epidemic.
“A growing number of people are obese – 650 million according to the World Health Organization – which not only has health consequences but is a serious financial burden for global health systems. These new findings suggest that losing weight is particularly important for healthy brain ageing or for recovery in people who suffer strokes or brain injuries, where learning is fundamental for recovery.”
— Dr Brenton Hordacre
[Source(s): University of South Australia, Newswise]
[ARTICLE] Virtual reality-based treatment for regaining upper extremity function induces cortex grey matter changes in persons with acquired brain injury – Full Text
Posted by Kostas Pantremenos in Paretic Hand, REHABILITATION, Virtual reality rehabilitation on September 14, 2020
Abstract
Background
Individuals with acquired brain injuries (ABI) are in need of neurorehabilitation and neurorepair. Virtual anatomical interactivity (VAI) presents a digital game-like format in which ABI survivors with upper limb paresis use an unaffected limb to control a standard input device and a commonplace computer mouse to control virtual limb movements and tasks in a virtual world.
Methods
In a prospective cohort study, 35 ambulatory survivors of ABI (25/71% stroke, 10/29% traumatic brain injury) were enrolled. The subjects were divided into three groups: group A received VAI therapy only, group B received VAI and physical/occupational therapy (P/OT), and group C received P/OT only. Motor skills were evaluated by muscle strength (hand key pinch strength, grasp, and three-jaw chuck pinch) and active range of motion (AROM) of the shoulder, elbow, and wrist. Changes were analyzed by ANOVA, ANCOVA, and one-tailed Pearson correlation analysis. MRI data was acquired for group A, and volumetric changes in grey matter were analyzed using voxel-based morphometry (VBM) and correlated with quantified motor skills.
Results
AROM of the shoulder, elbow, and wrist improved in all three groups. VBM revealed grey matter increases in five brain areas: the tail of the hippocampus, the left caudate, the rostral cingulate zone, the depth of the central sulcus, and the visual cortex. A positive correlation between the grey matter volumes in three cortical regions (motor and premotor and supplementary motor areas) and motor test results (power and AROM) was detected.
Conclusions
Our findings suggest that the VAI rehabilitation program significantly improved motor function and skills in the affected upper extremities of subjects with acquired brain injuries. Significant increases in grey matter volume in the motor and premotor regions of affected hemisphere and correlations of motor skills and volume in nonaffected brain regions were present, suggesting marked changes in structural brain plasticity.
Background
Neurological disorders, including acquired brain injuries (ABIs) are important causes of disability and death worldwide [1, 2]. Although age-standardized mortality rates for ischemic and hemorrhagic strokes have decreased in the past two decades, the absolute number of stroke survivors is increasing, with most of the burden in low- and middle-income countries [3]. Another major issue is that trends toward increasing stroke incidence at younger ages has been observed [4]. Moreover, this type of ABI is the leading cause of long-term disability in the United States, with an estimated incidence of 795,000 strokes yearly [2].
In more than 80% of stroke survivors, impairments are seen in at least one of the upper limbs. Six months after a stroke, 38% of patients recover some dexterity in the paretic arm, though only 12% recover substantial function even in spite of having received physical/occupational therapy (P/OT) [5]. Only a few survivors are able to regain some useful function of the upper limb. Failing to achieve useful function has highly negative impacts on the performance of daily living activities [6, 7]. Regaining control and improving upper limb motor function after ABIs are therefore crucial goals of motor system rehabilitation. In left-sided limb impairment, neglect syndrome can contribute to a worsened clinical state, making the alleviation of symptoms even more difficult to achieve. Mirror therapy has been reported as a promising approach to improve neglect symptoms [8, 9].
MRI has been used to track changes in brain connectivity related to rehabilitation [10], and several studies of healthy individuals playing off-the-shelf video games have demonstrated changes in the human brain resulting from interactions in a virtual world (VW) [11, 12]. Furthermore, playing video games results in brain changes associated with regaining improved, purposeful physical movements [13, 14]. The socio-cultural relevance of virtual reality (VR) and VW applications lies, more generally, in the fact that these technologies offer interactive environments to users. These interactive environments are actually present in the users’ experiences while less so in the world they share as biological creatures [15]. The way in which we engage with VWs allows for rehabilitation exercises and activities that feel similar to their actual physical world counterparts [11]. In the past two decades, researchers have demonstrated the potential for the interactive experiences of VWs to provide engaging, motivating, less physically demanding, and effective environments for ABI rehabilitation [9, 16,17,18].
One of the suitable rehabilitation methods seems to be exercises and tasks in VW called virtual anatomical interactivity (VAI) [19]. This method provides sensory stimulation / afferent feedback and allows the independent control of an anatomically realistic virtual upper extremity capable of simulating human movements with a true range of motion. ABI survivors are able to relearn purposeful physical movements and regain movement in their disabled upper extremities [19]. Contrary to conventional therapy, which exercises impaired upper limbs to improve limb movement, the general VAI hypothesis is that brain exercises alone (or combined with traditional therapy) may positively influence neuroplastic functions. In the VW, subjects can move their virtual impaired limbs using their healthy hands, meaning simulated physical movements are survivor-authored. Virtual visuomotor feedback may help regain functional connectivity between the brain and the impaired limb, therefore also regaining voluntary control of the limb.
The aim of the study was to test if the shoulder, elbow, and wrist movement; hand pinch strength; and grip strength of the paretic side improved through the use of VAI exclusively or combined with P/OT for upper extremities and how these approaches improved functional outcomes measured by the Action Reach Arm Test [20]. The relationship between changes in abilities to control upper extremities and volumetric changes in cortex grey matter measured by VBM and using MRI was also explored.[…]
Continue —-> https://jneuroengrehab.biomedcentral.com/articles/10.1186/s12984-020-00754-7

[ARTICLE] The Promotoer, a brain-computer interface-assisted intervention to promote upper limb functional motor recovery after stroke: a study protocol for a randomized controlled trial to test early and long-term efficacy and to identify determinants of response – Full Text
Posted by Kostas Pantremenos in Neuroplasticity, Paretic Hand on June 29, 2020
Abstract
Background
Stroke is a leading cause of long-term disability. Cost-effective post-stroke rehabilitation programs for upper limb are critically needed. Brain-Computer Interfaces (BCIs) which enable the modulation of Electroencephalography (EEG) sensorimotor rhythms are promising tools to promote post-stroke recovery of upper limb motor function. The “Promotoer” study intends to boost the application of the EEG-based BCIs in clinical practice providing evidence for a short/long-term efficacy in enhancing post-stroke hand functional motor recovery and quantifiable indices of the participants response to a BCI-based intervention. To these aims, a longitudinal study will be performed in which subacute stroke participants will undergo a hand motor imagery (MI) training assisted by the Promotoer system, an EEG-based BCI system fully compliant with rehabilitation requirements.
Methods
This longitudinal 2-arm randomized controlled superiority trial will include 48 first ever, unilateral, subacute stroke participants, randomly assigned to 2 intervention groups: the BCI-assisted hand MI training and a hand MI training not supported by BCI. Both interventions are delivered (3 weekly session; 6 weeks) as add-on regimen to standard intensive rehabilitation. A multidimensional assessment will be performed at: randomization/pre-intervention, 48 h post-intervention, and at 1, 3 and 6 month/s after end of intervention. Primary outcome measure is the Fugl-Meyer Assessment (FMA, upper extremity) at 48 h post-intervention. Secondary outcome measures include: the upper extremity FMA at follow-up, the Modified Ashworth Scale, the Numeric Rating Scale for pain, the Action Research Arm Test, the National Institute of Health Stroke Scale, the Manual Muscle Test, all collected at the different timepoints as well as neurophysiological and neuroimaging measures.
Discussion
We expect the BCI-based rewarding of hand MI practice to promote long-lasting retention of the early induced improvement in hand motor outcome and also, this clinical improvement to be sustained by a long-lasting neuroplasticity changes harnessed by the BCI-based intervention. Furthermore, the longitudinal multidimensional assessment will address the selection of those stroke participants who best benefit of a BCI-assisted therapy, consistently advancing the transfer of BCIs to a best clinical practice.
Trial registration
Name of registry: BCI-assisted MI Intervention in Subacute Stroke (Promotoer).
Trial registration number: NCT04353297; registration date on the ClinicalTrial.gov platform: April, 15/2020.
Background
Stroke is a major public health and social care concern worldwide [1]. The upper limb motor impairment commonly persists after stroke, and it represents the major contribution to long-term disability [2]. It has been estimated that the main clinical predictor of whether a patient would come back to work is the degree of upper extremity function [3]. Despite the intensive rehabilitation, the variability in the nature and extent of upper limb recovery remains a crucial factor affecting rehabilitation outcomes [4].
Electroencephalography (EEG)-based Brain-Computer Interface (BCI) is an emerging technology that enables a direct translation of brain activity into motor action [5]. Recently, EEG-based BCIs have been recognized as potential tools to promote functional motor recovery of upper limbs after stroke (for review see [6]). Several randomized controlled trials have shown that stroke patients can learn to modulate their EEG sensorimotor rhythms [7] to control external devices and this practice might facilitate neurological recovery both in subacute and chronic stroke phase [8,9,10].
We were previously successful in the design and validation of an EEG sensorimotor rhythms–based BCI combined with realistic visual feedback of upper limb to support hand motor imagery (MI) practice in stroke patients [11, 12]. Our previous pilot randomized controlled study [8] with the participation of 28 subacute stroke patients with severe motor deficit, suggested that 1 month BCI-assisted MI practice as an add-on intervention to the usual rehabilitation care was superior with respect to the add-on, 1 month MI training alone (ie., without BCI support) in improving hand functional motor outcomes (indicated by the significantly higher mean score at upper extremity Fugl-Meyer scale in the BCI with respect to control group). A greater involvement of the ipsilesional hemisphere, as reflected by a stronger motor-related EEG oscillatory activity and connectivity in response to MI of the paralyzed trained hand was also observed only in the BCI-assisted MI training condition. These promising findings corroborated the idea that a relatively low-cost technique (i.e. EEG-based BCI) can be exploited to deliver an efficacious rehabilitative intervention such as MI training and prompted us to undertake a translational effort by implementing an all-in-one BCI-supported MI training station– the Promotoer [13].
Yet, important questions remain to be addressed in order to improve the clinical viability of BCIs such as defining whether the expected early improvements in functional motor outcomes induced by the BCI-assisted MI training in subacute stroke [8] can be sustained in a long-term as it has been shown for other BCI-based approaches in chronic stroke patients [10, 14]. This requires advancements in the knowledge on brain functional re-organization early after stroke and on how this re-organization would correlate with the functional motor outcome (evidence-base medicine). Last but not least, the definition of the determinants of the patients response to treatment is paramount to optimize the process of personalized medicine in rehabilitation. We will address these questions by carrying out a randomized trial to eventually establish the fundamentals for a cost-effective use of EEG-based BCI technology to deliver a rehabilitative intervention such as the MI in hospitalized stroke patients.
Aim and hypotheses
The “Promotoer” study is a randomized controlled trial (RCT) designed to provide evidence for a significant early improvement of hand motor function induced by the BCI-assisted MI training operated via the Promotoer and for a persistency (up to 6 months) of such improvement. Task-specific training was reported to induce long-term improvements in arm motor function after stroke [15,16,17]. Thus, our hypothesis is that the BCI-based rewarding of hand MI tasks would promote long-lasting retention of early induced positive effect on motor performance with respect to MI tasks practiced in an open loop condition (ie, without BCI). Accordingly, the primary aim of the “Promotoer” RCT will be first to determine whether the BCI based intervention (MI-BCI) administered by means of a BCI system fully compatible with a clinical setting (the Promotoer), is superior to a non-BCI assisted MI training (MI Control) in improving hand motor function outcomes in sub-acute stroke patients admitted to the hospital for their standard rehabilitation care; secondly, we will test whether the efficacy of BCI-based intervention on hand motor function outcomes is sustained long-term after the end of intervention (6 months follow-up). A further hypothesis is that such clinical improvement would be sustained by a long-lasting neuroplasticity changes as harnessed by the BCI–based intervention. This hypothesis rises from current evidence for an early enhancement of post-stroke plastic changes enabled by BCI-based trainings [8,9,10]. To test this hypothesis, a longitudinal assessment of the brain network organization derived from advanced EEG signal processing (secondary objective) will be performed.
The heterogeneity of stroke makes prediction of treatment responders a great challenge [18]. The potential value of a combination of neurophysiological and neuroimaging biomarkers with the clinical assessment in predicting post-stroke motor recovery has been recently highlighted [19]. Our hypothesis is that the longitudinal combined functional, neurophysiological and neuroimaging assessment over 6 months from the intervention will allow for insights into biomarkers and potential predictors of patients response to the BCI-Promotoer training (secondary aim). To this purpose, well-recognized factors contributing to recovery after stroke such as the relation between clinical profile, lesion characteristics and patterns of post-stroke motor cortical re-organization (eg., ipsilesional/contralesional primary and non-primary motor areas, cortico-spinal tract integrity, severity of motor deficits at baseline; for review see [19]) will be taken into account.[…}


