If you or a loved one has suffered from a stroke, there are many difficulties that can develop as a result. Primarily, these effects are physical, emotional, and cognitive.
Below, we provide tips on how to overcome these common post-stroke conditions. Keep in mind that dealing with the aftermath of a stroke can be frustrating, but with patience and consistent effort, considerable progress can be made.
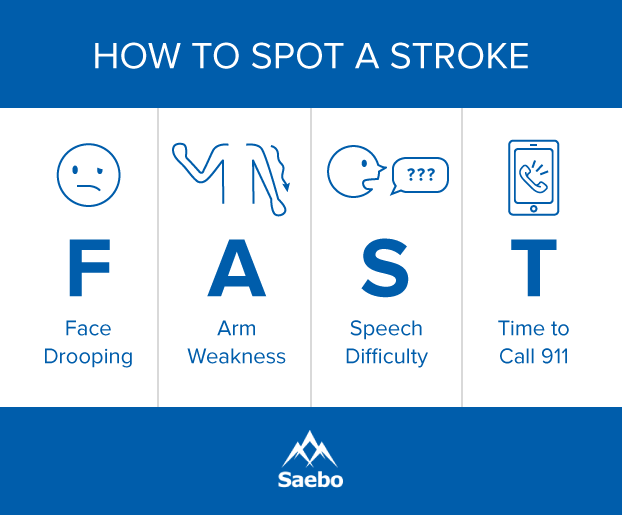
Tip 1. Recognize Symptoms of Stroke
One of the most important ways to successfully recover from stroke, is by taking preventative measures such as knowing and recognizing the symptoms of a stroke because immediate treatment can be life saving and greatly affects the chances for a full recovery. Unfortunately the chances of a second stroke occurring increases in stroke survivors. According to The National Stroke Association, about 25% of stroke survivors will experience a second stroke. Within the first 5 years after the first stroke, risk of a second stroke is about 40% higher. Fortunately it is estimated that of all secondary strokes, about 80% of them are preventable with lifestyle changes and medical intervention. Read more about recognizing the symptoms of stroke in men and in women to better prepare you to act FAST.

Tip 2. Walking Again and Foot Drop
Foot drop is the difficulty or inability to lift the front part of the foot because of fatigue or damage affecting the muscles and nerves that aid in its movement. To combat this, using a brace or Ankle-foot Orthoses (AFO) has proven to be a major aid in rehabilitation. These devices prevent the front of the foot from dipping down and disrupting walking movements.
Leg exercises described in this supplementary post after experiencing a stroke are crucial for recovery. While each patient should have a custom exercise routine, personalized for you, there are several exercises that should be included in most every stroke survivor’s regimen. These low-impact strength and stretching leg exercises for stroke recovery are a good complement to use in conjunction with the Saebo MyoTrac Infiniti biofeedback system.
Richard Sealy, director of The Rehab Practice, a private neuro-therapy rehabilitation program in the United Kingdom, regularly works with individuals, families, and caregivers to establish custom exercise routines to aid in recovery from long-term neurological problems, like the damage caused by stroke. While he acknowledges that each patient should have a custom exercise routine specific and personal to their struggles, he recommends a series of exercises for anyone working to strengthen their legs and improve range of motion during stroke recovery.
Rehabilitation of the legs and feet can occur at a faster rate with a combination of the aforementioned exercises and orthopedic aids such as the SaeboStep.The SaeboStep is a unique foot drop brace worn on the outside of the shoe that assists with lifting the toes when walking. It is made to eliminate cumbersome, unreliable splints and braces placed within the shoe.
Tip 3. Dealing with Curled Toes
Often referred to as “curled toes” or “claw toe,” this symptom is caused by a miscommunication between the brain and muscles within the foot. This misfiring of signals causes an issue with controlling muscular movements, leading to over-contracting of the toes and spasticity, a condition where there is a miscommunication between the brain and the muscles in the toes, causing them to over contract.
The best way to regain strength and movement while dealing with this condition is to create a routine with a variety of exercises—toe taps, floor grips, finger squeezes, and toe-extensor strengthening. With effort and repetition, these workouts can make a huge difference in recovery.
Tip 4. Lack of Arm Function
One of the most common deficiencies following a stroke is the impairment of the arm and hand. This typically results in decreased strength, coordination, and range of motion. Those affected are often unable to support their own arms in order to perform rehabilitation exercises. When this occurs it is crucial that you include additional arm support during rehabilitation to avoid the arms becoming weaker due to learned non-use.
Learned non-use occurs when a stroke survivor prefers to use their strong arm because it is easier to move. This tendency makes it even more difficult for a stroke survivor to recover, because challenging the weakened arm with these exercises plays a crucial role in regaining arm function. Often physical therapists and occupational therapists use a technique known as Constraint-Induced Movement Therapy (or CIMT) to challenge a weakened shoulder and make further exercises and drills possible. Mobile arm supports such as the SaeboMAS and SaeboMAS mini help support the weight of the arm, allowing the user to do a much wider range of exercises. For more information about the SaeboMAS and how it can aid in stroke recovery click here.
As with rehabilitating any part of the body with reduced function after a stroke, it is important to consistently repeat the exercises and stretches to strengthen the brain-muscle connections. It is also important to stay positive and try to have fun with your rehab. Here are 35 fun rehab activities for stroke patients to help keep you motivated.
Tip 5. Hand Paralysis
Paralysis is the inability of a muscle to move voluntarily. The National Stroke Association sites as many as 9 out of 10 stroke survivors have some degree of paralysis following a stroke. Rehabilitation and therapy can help to regain voluntary movement, even several years after the stroke takes place.
The primary symptoms of hand paralysis are spasticity (stiff muscles), weakness, and lack of coordination. Fortunately, there are several methods of treatment in addition to therapy to help manage and recover from spasticity. Additional treatments include medications to relax muscles, botox injections (relaxes muscles temporarily), stretching exercises, anti-spasticity orthotics, and functional orthoses. Surgery is another option in the most severe cases.
The least invasive and most permanent treatment for hand paralysis is therapy to rehabilitate the connection between your brain and muscles using neuroplasticity. To make these exercises even more effective and to increase your rate of recovery, it is important to repeat your hand exercises often. By performing exercises repeatedly, you are strengthening that brain-muscle connection.
Tip 6. Difficulty Speaking and Communicating
Another common side effect of stroke is aphasia, which is the inability to speak or understand speech. This is one of the most frustrating side effects for survivors to deal with. It’s estimated that 25 to 40 percent of people who suffer from a stroke develop aphasia, though this condition is not limited to stroke survivors. Aphasia occurs when there is damage to the brain, specifically the left side that deals with language. There are two primary forms of aphasia: receptive aphasia and expressive aphasia. Receptive aphasia is when the individual has trouble understanding what is being said to them. Expressive aphasia is when the individual is having difficulty expressing what they want to say.
When communicating with someone with receptive aphasia, try not to use long complex sentences. When communicating with someone with expressive aphasia, it is important to be patient and remember that the person’s intelligence has not been affected by the stroke, just their ability to speak.
For those with aphasia, the most important thing you can do to improve your communication is to take a deep breath and try to relax. If you can remain relaxed and focus on what you are trying to say you will have much greater success. It is easy to get flustered or feel self conscious, but you shouldn’t. Create tools that you can use to make communication easier such as a book of words, pictures, phrases, or symbols that can help you get your message across. If you are going out and know you will not be around friends or family, it may also be helpful to carry a card or piece of paper that indicates that you have aphasia and explains what it is, just in case you find yourself needing to explain your condition.
Once these tools are set in place, seeking the help of a speech-language pathologist (SLP) can greatly increase one’s ability to regain normal speech behavior. SLPs can assist in rehabilitating all types of physical speech behavior and offer support and proper guidance for you or a loved one. Read more about aphasia and recovery here.
Tip 7: Coping with PTSD After Stroke
Following a stroke, it is fairly common for a survivor to experience PTSD, or Post-Traumatic Stress Disorder. This condition is usually associated with combat veterans and sexual-assault survivors; however, according to a study published in the journal PLoS One, almost a quarter of stroke survivors experience some form of PTSD.
Common symptoms of PTSD include the victim experiencing the traumatic event over and over in their head or in the form of nightmares. This replaying of the event is typically accompanied by the individual’s unyielding anxiety and feelings of self doubt or misplaced guilt over their condition. Some experience a state of hyperarousal or feelings of being overly alert.
The two main treatments for PTSD include medications such as antidepressants, anti-anxiety medications or psychotherapy. If you are experiencing PTSD, it is important that you communicate how you feel with your doctor, family, and friends, as a strong support system can help you find the relief from psychological pain that you deserve.
Tip 8: Understanding Fatigue
Feeling tired is a normal part of life for everyone, but for stroke survivor, fatigue is a very common symptom that can be frustrating to deal with. About 40 to 70 percent of stroke survivors experience fatigue, which can make recovering feel even more difficult. Post-stroke fatigue is draining both physically and emotionally/mentally, and rest may not be the only solution.
It is important to discuss the fatigue with a doctor so they can rule out potential medical causes or determine if fatigue might stem from current medications. By speaking with the proper medical professionals and taking time to squeeze in a nap or rest as often as possible—and by maintaining a positive mindset—you can help yourself or a loved one combat the constant drowsiness of fatigue and work on returning to pre-stroke energy levels. The key thing to realize is that some level of post-stroke fatigue is normal and that survivors need to be proactive about treating and working around it.
Tip 9: Counteract Learned Non-Use
If the limbs weakened after stroke are not consistently exercised over time, muscles have the potential to atrophy—waste away due to cell degeneration. This often occurs when the person tries to compensate for their weak limb by using their stronger limb more often. Daily attempts to move the affected limbs are necessary to maintain and improve functionality.One method is the use of Constraint-Induced Movement Therapy (CIMT). CIMT is a form of therapy that prevents the unaffected limbs from moving while trying to exercise the affected ones.
Tip 10: Reduce Inflammation and Stress
Inflammation in the body can cause other issues to arise, which is why it’s important to stay stress free whenever possible. When stress does begin to take hold, a hormone called cortisol floods the body, causing pH levels to become imbalanced with acidity. High acidity levels—after an extended period of time—can kill good bacteria in the body while giving rise to bad bacteria, ultimately weakening the immune system.
With that in mind, a natural probiotic like yogurt is a great way to boost good bacteria in the body. Supplemental drinks can also improve the immune system significantly. In addition to pH balance, adopting stress management exercises such as yoga, deep breathing, tai chi, qi gong, and meditation, can limit one’s cortisol levels, promoting overall health.
Tip 11: Coping with Emotional Effects
Experiencing a stroke is not only a major hardship to overcome physically; it can also take a huge toll on a survivor’s emotions in many ways.
If the area of your brain that controls personality or emotion is affected, you may be susceptible to changes in your emotional response or everyday behavior. Strokes may also cause emotional distress due to the suddenness of their occurrence. As with any traumatic life experience, it may take time for you or your loved one to accept and adapt to the emotional trauma of having experienced a stroke.
Some common emotional changes strokes may cause are PseudoBulbar Affect, depression, and anxiety. Thankfully, there are several methods for treating the emotional changes associated with a stroke, with the first step being to discuss how you or your loved one is feeling with a doctor. Treatment may consist of one, or a combination, of the following: one-on-one counseling, group counseling, medication, diet, and exercise. The most effective treatment is different for everyone, so it is important to discuss and explore which combination works best for your or your loved one.
PseudoBulbar Affect
Sometimes referred to as “reflex crying,” “emotional lability,” or “labile mood,” PseudoBulbar Affect (PBA) is a symptom of damage to the area of the brain that controls expression of emotions, and it is one of the most frequently reported post-stroke behaviors. Characteristics of the disorder include rapid changes in mood, such as suddenly bursting into tears and stopping just as suddenly or even beginning to laugh at inappropriate times.
Depression
Survivors have a one in four chance of developing serious depression as a side effect of stroke. If you are feeling sad, hopeless, or helpless after having suffered a stroke, you may be experiencing this. Other symptoms of depression may include irritability or changes to your eating and sleeping habits. Talk to your doctor if you are experiencing any of these symptoms, as it may be necessary to treat with prescription antidepressants or therapy to avoid it becoming a road block to your recovery.
Along with medication and therapy, a lot of research shows that a few simple lifestyle changes help relieve the symptoms of depression. If you or a loved one is having a difficult time coping with the emotional repercussions of a stroke, here are tips on how to implement positivity and resilience:
- Attend a support group. Talking about your struggles with people in the same situation makes you feel less lonely and can offer helpful insight or different approaches to dealing with difficulties.
- Eat healthy food. A good diet is important for your general health and your recovery from stroke and can also improve your mental health.
- Remain socially active. Although you may not be able to do as much as you used to, it’s crucial to stay in touch with family and friends and take part in social activities.
- Be as independent as possible. Humans need to feel independent and competent. Stroke recovery may require the help of caregivers, but if there are things that you can safely do by yourself, insist on it.
- Exercise regularly. Physical activity, especially a low-impact one like walking, is proven to boost mental health and will also contribute to your recovery.

Tip 12: Set Recovery Goals with Your Therapist
Setting specific and meaningful goals can help keep one focused and motivated once they are achieved, and these goals can range from simple tasks to long-term accomplishments. By establishing a list consisting of difficulties and goals, overcoming obstacles can be put within reach.
When setting these goals, working with a therapist, doctor, or close friend can be a good way to find encouragement, as well as assistance in creating a list that places goals into an appropriate timeframe. Overall, a therapist will be familiar with your case, understanding the issues and complications, and will be able to offer sound advice in all aspects of recovery.
Tip 13: Stay Motivated
Since apathy is common during stroke recovery, staying motivated can be a challenge. Combining one’s interests with a solid rehabilitation regimen can effectively eradicate feelings of lethargy and depression. The best thing to do is to focus on a reason for recovery and to associate it with your plan of action. This can be done by implementing sentimental items into daily routines, thus giving you personal and motivational support at all times. For example, if one of your routines is to write a list of things to do for the day, try writing it on the back of a special photo. That way, as you’re checking things off, you’ll have a little reminder to keep you motivated.
Tip 14: Watch Out For The Recovery Plateau Stage
The recovery plateau stage refers to the point at which a stroke survivor begins to see a slow down or stop in the progression in their recovery. Some of the most significant improvements often occur in the subacute phase, which is usually the first three to six months after the stroke (though there is anecdotal evidence of people making significant stroke recovery progress outside of that zone.)
Seeing improvement in the early days of a survivor’s recovery can make it a lot easier for them to stay motivated and continue working hard in therapy. Research shows that further recovery is still very possible after the plateau stage though, which is why it is so important to have a strong support system to encourage you to continue with therapy and working on recovery.
Tip 15: Working After Stroke
Since the brain is a major organ affected when it comes to strokes, chances are that some of its functions may have trouble performing like they did before. After a stroke, learning new things, or even just recalling information can be a challenge, and working through thoughts may suddenly be difficult.
After rehabilitation, many stroke survivors do find themselves able to return to work, but preparing for this transition can come with a lot of questions. Are you physically going to be able to perform your job? Will your disability benefits lapse? What do you need to communicate with your employer? These can be tough questions, but they do have answers. Some may not ever be able to go back to the same work, but for others, just a little assistance is needed.
When you are ready to return to work, it is important to know your rights and what your employer is, and is not, legally required to provide to employees with disabilities. Keep in mind that if you are unable to perform the essential functions of your job even with reasonable accommodation, your employer is not obligated to offer you a different position or create a new role for you. They might be willing to anyway, but it is not a requirement.
Tip 16: Understand and Combat Memory Loss
Not only is it common for stroke survivors to experience, but memory loss can affect a wide range of people through multiple factors. Age, physical trauma, and emotional stress have the potential to cause memory decline, but rebuilding memory’s strength is highly possible and can be fun.
Specifically, incorporating technology into daily rehabilitation exercises is a great way to show quick improvements. There are numerous apps for smartphones and tablets that use different techniques to significantly improve memory, and they have the ability to set reminders, schedule appointments, and oversee other illnesses.
Tip 17: Be Aware of Vascular Dementia
A common problem among stroke survivors, this symptom disrupts cognitive functions, which can make it challenging for one to sort out information.
Due to the damage of blood vessels from a stroke, blood pressure, cholesterol, and blood sugar must be maintained at healthy levels to ensure good blood flow throughout the body. If you are diabetic, it is crucial that you are paying careful attention to your blood sugar and insulin levels. Studies have shown that by managing these three components, vascular dementia can be decreased or prevented.
Helping Stroke Survivors Help Themselves
The process of stroke recovery is long and full of ups, downs, twists, and turns. It takes hard work and dedication to regain mental and physical function after a stroke. The information and tips above will help you to identify and overcome the many challenges that come with recovery.
To read our answers to the most common stroke recovery questions, click here. And remember, at the end of the day, there are dozens of approaches you can take to improve the speed of stroke recovery.
All content provided on this blog is for informational purposes only and is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition. If you think you may have a medical emergency, call your doctor or 911 immediately. Reliance on any information provided by the Saebo website is solely at your own risk.
via 17 Ways To Help Stroke Survivors Recover Faster | Saebo