Posts Tagged REHABILITATION
[Abstract] Evaluation of custom-made VR exergame for at-home Stroke rehabilitation. A longitudinal single-arm study. – Full Text PDF
Posted by Kostas Pantremenos in Paretic Hand, Video Games/Exergames, Virtual reality rehabilitation on April 26, 2024
Abstract
Exercise games (Exergames) based on Virtual Reality (VR) have emerged as a promising option for supporting physical rehabilitation in stroke users. As a com- plementary therapy, they offer valuable benefits such as therapy engagement and enjoyment. In this study, we assessed the effectiveness of an immersive, custom- made VR exergame designed for upper limb rehabilitation in stroke participants aged 50 and above. We conducted 14 sessions of 15 minutes involving ten par- ticipants (6 females, ages 58.1 ± 7.5 years old) who volunteered to participate in an assisted at-home rehabilitation process. The study employed a range of evaluation tests to measure physical rehabilitation and game user experience out- comes. The tests included pre- and post-assessments of range of motion (ROM), the Ashworth spasticity test, and the Borg rating of perceived fatigue question- naire. To evaluate the game participant experience, we used the VR Neuroscience Questionnaire (VRNQ), and the Immersive Tendencies Questionnaire (ITQ). Our results revealed significant improvements in the range of motion for elbow and shoulder flexion, extension, adduction, and abduction. Furthermore, we observed a reduction in Ashworth spasticity, and the fatigue scale showed reduced per- ception comparing the last with the first session, although the difference was insignificant. The VRNQ questionnaire indicated significant enhancements in the domains related to ”Game Experience” and ”Game Mechanics” and an overall reduction of the perceived “Motion Sickness”. In the ITQ questionnaire, partic- ipants reported high levels of ”Attention,” and while there were no significant differences in ”Immersion” and ”Enjoyment,” a considerable improvement was observed in ”Excitement”. In summary, our results indicate that the immersive VR exergame improved the range of motion, spasticity, and overall game user experience among participants with stroke in a longitudinal, single-arm inter- vention. We conclude that using custom-made VR exergames is an effective and motivating tool for upper limb rehabilitation, with positive changes in both clin- ical and perception outcomes, and the positive and measurable effects persist after the first sessions. These findings support using VR exergames as a comple- mentary tool for at-home rehabilitation therapy with good ease of use, improved physical rehabilitation outcomes, and high treatment adherence.
[Abstract] Evaluation of custom-made VR exergame for at-home Stroke rehabilitation. A longitudinal single-arm study. – Full Text PDF
Posted by Kostas Pantremenos in Tele/Home Rehabilitation, Video Games/Exergames, Virtual reality rehabilitation on April 21, 2024
Abstract
Exercise games (Exergames) based on Virtual Reality (VR) have emerged as a promising option for supporting physical rehabilitation in stroke users. As a com- plementary therapy, they offer valuable benefits such as therapy engagement and enjoyment. In this study, we assessed the effectiveness of an immersive, custom- made VR exergame designed for upper limb rehabilitation in stroke participants aged 50 and above. We conducted 14 sessions of 15 minutes involving ten par- ticipants (6 females, ages 58.1 ± 7.5 years old) who volunteered to participate in an assisted at-home rehabilitation process. The study employed a range of evaluation tests to measure physical rehabilitation and game user experience out- comes. The tests included pre- and post-assessments of range of motion (ROM), the Ashworth spasticity test, and the Borg rating of perceived fatigue question- naire. To evaluate the game participant experience, we used the VR Neuroscience Questionnaire (VRNQ), and the Immersive Tendencies Questionnaire (ITQ). Our results revealed significant improvements in the range of motion for elbow and shoulder flexion, extension, adduction, and abduction. Furthermore, we observed a reduction in Ashworth spasticity, and the fatigue scale showed reduced per- ception comparing the last with the first session, although the difference was insignificant. The VRNQ questionnaire indicated significant enhancements in the domains related to ”Game Experience” and ”Game Mechanics” and an overall reduction of the perceived “Motion Sickness”. In the ITQ questionnaire, partic- ipants reported high levels of ”Attention,” and while there were no significant differences in ”Immersion” and ”Enjoyment,” a considerable improvement was observed in ”Excitement”. In summary, our results indicate that the immersive VR exergame improved the range of motion, spasticity, and overall game user experience among participants with stroke in a longitudinal, single-arm inter- vention. We conclude that using custom-made VR exergames is an effective and motivating tool for upper limb rehabilitation, with positive changes in both clin- ical and perception outcomes, and the positive and measurable effects persist after the first sessions. These findings support using VR exergames as a comple- mentary tool for at-home rehabilitation therapy with good ease of use, improved physical rehabilitation outcomes, and high treatment adherence.
[ARTICLE] Noninvasive spinal stimulation improves walking in chronic stroke survivors: a proof-of-concept case series – Full Text
Posted by Kostas Pantremenos in Gait Rehabilitation - Foot Drop on April 8, 2024
Abstract
Background
After stroke, restoring safe, independent, and efficient walking is a top rehabilitation priority. However, in nearly 70% of stroke survivors asymmetrical walking patterns and reduced walking speed persist. This case series study aims to investigate the effectiveness of transcutaneous spinal cord stimulation (tSCS) in enhancing walking ability of persons with chronic stroke.
Methods
Eight participants with hemiparesis after a single, chronic stroke were enrolled. Each participant was assigned to either the Stim group (N = 4, gait training + tSCS) or Control group (N = 4, gait training alone). Each participant in the Stim group was matched to a participant in the Control group based on age, time since stroke, and self-selected gait speed. For the Stim group, tSCS was delivered during gait training via electrodes placed on the skin between the spinous processes of C5–C6, T11–T12, and L1–L2. Both groups received 24 sessions of gait training over 8 weeks with a physical therapist providing verbal cueing for improved gait symmetry. Gait speed (measured from 10 m walk test), endurance (measured from 6 min walk test), spatiotemporal gait symmetries (step length and swing time), as well as the neurophysiological outcomes (muscle synergy, resting motor thresholds via spinal motor evoked responses) were collected without tSCS at baseline, completion, and 3 month follow-up.
Results
All four Stim participants sustained spatiotemporal symmetry improvements at the 3 month follow-up (step length: 17.7%, swing time: 10.1%) compared to the Control group (step length: 1.1%, swing time 3.6%). Additionally, 3 of 4 Stim participants showed increased number of muscle synergies and/or lowered resting motor thresholds compared to the Control group.
Conclusions
This study provides promising preliminary evidence that using tSCS as a therapeutic catalyst to gait training may increase the efficacy of gait rehabilitation in individuals with chronic stroke.
Trial registration NCT03714282 (clinicaltrials.gov), registration date: 2018-10-18.
Background
Stroke is the leading cause of adult-onset disability [1]. Despite many advances in gait research in the last decade, about 35% of stroke survivors fail to regain independence in performing activities of daily living due to the impaired function of their affected leg, and about 70% have gait deficits, including reduced walking speeds, asymmetrical walking patterns, and motor coordination issues [2,3,4].
Walking deficits after stroke mostly derive from a disruption of the corticospinal pathways that play an important role in transmitting sensory–motor commands [5, 6]. To address this, most interventions using non-invasive electrical pulses focus on stimulation of the motor cortex to activate dormant or new pathways [2, 7, 8]. However, while supra-spinal regions can facilitate fine locomotor control, spinal networks ultimately generate the basic locomotor pattern [9, 10]. More interestingly, a recent study using functional MRI showed increased blood-oxygen-level dependent activities in motor cortex following transcutaneous spinal cord stimulation (tSCS) in individuals with stroke [11]. Therefore, we hypothesized that tSCS would facilitate an improvement of gait after stroke. Our previous work, in collaboration with additional researchers, established anatomical and physiological changes in the spinal cord after stroke [12, 13], offering a theoretical basis for testing our hypothesis of targeting the spinal circuits for post-stroke recovery.
Recently, Moshonkina et al. reported functional improvements in post-stroke individuals after 2 weeks of tSCS with standard physical therapy, achieving the minimum clinical important differences (MCID) in the 6 min walk test and comfortable walking speed [14]. The same investigators reported immediate improvements in walking kinematics after a single tSCS session [15, 16]. Notably, however, none of the studies mentioned above investigated the effects of more than 4 weeks of training nor tried to explore the potential neurophysiological differences accompanied with gait outcomes. Consequently, it remains unclear whether tSCS can exert a lasting impact on restoration of function following a stroke.
We investigated whether tSCS combined with symmetry-focused gait training has a sustained effect on gait recovery after chronic stroke. We hypothesized that longer-term gait training (24 sessions) with tSCS would lead to greater sustained improvements in walking function compared to control treatment focused solely on gait training. Specifically, we focused on gait symmetry since such improvements can have lasting effects on balance and overall mobility of stroke survivors [6]. We also expected that gait improvements would be associated with physiological changes in muscle coordination measured from electromyography (EMG) of the paretic side, and spinal excitability determined by the spinal motor evoked responses (sMERs). […]
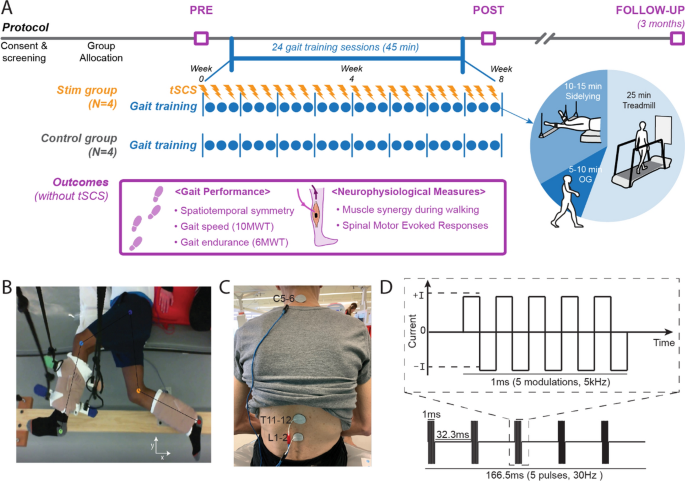
Study protocol and stimulation setup. A Overall experimental protocol. B Top–down view of position of the legs extended beyond the edge of the table and supported with vertically cables during the side-lying training of a participant (Stim 2). C tSCS delivered using surface electrodes on the skin between the C5–6, T11–12, and L1–2 spinous processes (cathode) and a surface electrode on each anterior crest (anode, not shown). D Schematic representation of biphasic pulse sequence used for tSCS. tSCS transcutaneous spinal cord stimulation, OG overground walking, 10MWT 10-m walk test, 6MWT 6-min walk test
[Preprint] Feasibility of Simultaneous Transcranial Direct Current Stimulation During Gait Training in Chronic Stroke Patients: A Randomized Double-blind Pilot Clinical Trial
Posted by Kostas Pantremenos in Gait Rehabilitation - Foot Drop, tDCS/rTMS on April 2, 2024
Abstract
Background
Transcranial direct current stimulation (tDCS) is a therapeutic tool for improving post-stroke gait
disturbances, with ongoing research focusing on specific protocols for its application. We evaluated the
feasibility of a rehabilitation protocol that combines tDCS with conventional gait training.
Methods
This was a randomized, double-blind, single-center pilot clinical trial. Patients with unilateral hemiplegia
due to ischemic stroke were randomly assigned to either the tDCS with gait training group or the sham
stimulation group. The anodal tDCS electrode was placed on the tibialis anterior area of the precentral
gyrus while gait training proceeded. Interventions were administered 3 times weekly for 4 weeks.
Outcome assessments, using the 10-meter walk test, Timed Up and Go test, Berg Balance Scale,
Functional Ambulatory Scale, Modified Barthel Index, and EQ-5D-3L, were conducted before and after the
intervention and again at the 8-week mark following its completion. Repeated-measures ANOVA was used for comparisons between and within groups.
Results
Twenty-six patients were assessed for eligibility, and 20 were enrolled and randomized. No significant
differences were observed between the tDCS with gait training group and the sham stimulation group in
gait speed after the intervention. However, the tDCS with gait training group showed significant
improvement in balance performance in both within-group and between-group comparisons. In the
subgroup analysis of patients with elicited motor-evoked potentials, comfortable pace gait speed
improved in the tDCS with gait training group. No serious adverse events occurred throughout the study.
Conclusions
Simultaneous tDCS during gait training is a feasible rehabilitation protocol for chronic stroke patients
with gait disturbances.
Introduction
Impairment of independent gait is one of the most disabling consequences after a stroke [1]. Gait
abnormality in stroke patients arises from a complex interplay of factors, including lower limb motor
weakness and decreased balance. Some individuals may not be entirely incapable of walking, but they
may still require gait aids or assistance from caregivers. Gait disturbances pose a greater risk of further
injury due to falls. It is well known that fall-associated fractures result in significant socioeconomic costs
[2]. Additionally, gait disturbances lead to limitations in social activity, thereby reducing the quality of life
of stroke patients. Therefore, improving gait performance in stroke patients has long been a desire shared
by patients and physicians.
Transcranial direct current stimulation (tDCS) is a non-invasive brain stimulation technique that aims to
modulate the human brain by delivering low-intensity electrical current through the scalp. The mechanism of tDCS is explained by 2 principles: (1) the enhancement of cortical activity through a polarity shift in the resting membrane potential and (2) the upregulation of neural plasticity through long-term potentiation [3]. Applying anodal tDCS to patients with subacute stroke has been associated with beneficial effects on motor function [4]. However, inconsistent results have emerged across studies, with some failing to observe significant improvements in patients who underwent tDCS compared to sham stimulation [5].
Additionally, there has been substantial variability in factors, such as stimulation area, intensity, duration,
and the number of sessions among different tDCS protocols, highlighting the need for further research to
establish an optimal tDCS protocol for maximizing the effect of conventional gait training methods.
Studies focusing on stimulation timing suggest that combining tDCS with gait training simultaneously
shows more promising results in improving gait performance compared to protocols that administer gait
training and tDCS separately [6].
This study aimed to evaluate the feasibility of a rehabilitation protocol that combines simultaneous tDCS
with conventional gait training and to investigate its impact on gait performance in chronic stroke
patients.[…]
[ARTICLE] Stroke patients’ motivation for home-based upper extremity rehabilitation with eHealth tools – Full Text
Posted by Kostas Pantremenos in Paretic Hand, REHABILITATION, Tele/Home Rehabilitation on February 18, 2024
Abstract
Purpose
eHealth-based exercise therapies were developed to increase stroke patients’ adherence to home-based motor rehabilitation. However, these eHealth tools face a rapid decrease in use after a couple of weeks. This study investigates stroke patients’ motivation for home-based upper extremity rehabilitation with eHealth tools and their relation with Basic Psychological Needs.
Materials and methods
This is a qualitative study using thematic analysis. We conducted semi-structured interviews with stroke patients with upper extremity motor impairments, who were discharged home from a rehabilitation centre, after they interacted with a novel eHealth coach demonstrator in their homes for five consecutive days.
Results
We included ten stroke patients. Thematic analysis resulted in eight themes for home-based rehabilitation motivation: Curiosity, Rationale, Choice, Optimal challenge, Reference, Encouragement, Social Support and Trustworthiness. Those themes are embedded into three Basic Psychological Needs: “Autonomy”, “Competence”, and “Relatedness”.
Conclusion
Eight motivational themes related to the three Basic Psychological Needs describe stroke patients’ motivation for home-based upper extremity rehabilitation. We recommend considering those themes when developing a home-based eHealth intervention for stroke patients to increase the alignment of eHealth tools to the patient’s needs and reduce motivational decreases in home-based rehabilitation.
Implications for rehabilitation
- Stroke patients show motivational decreases and decreased use of eHealth tools in home-based rehabilitation after a couple of weeks.
- Eight motivational themes describe home-based rehabilitation motivation in stroke patients: Curiosity, Rationale, Choice, Optimal challenge, Reference, Encouragement, Social Support and Trustworthiness.
- Those themes are embedded into three Basic Psychological Needs: “Autonomy”, “Competence”, and “Relatedness”.
- Those themes should be considered when developing a home-based eHealth intervention for stroke patients to increase the alignment of eHealth tools to the patient’s needs and reduce motivational decreases in home-based rehabilitation.
Introduction
Over two-thirds of stroke survivors experience upper extremity (UE) motor impairment, limiting capacity and resulting in significant daily functioning limitations and decreased quality of life [Citation1]. Rehabilitation programs involve stimulation of UE use and intensive UE exercises (e.g. a high number of repetitions per time unit) [Citation2]. Adherence to intensive daily UE use and exercise is essential to optimise UE capacity and daily life functioning [Citation3–5]. Exercise adherence may be specifically challenging for patients discharged from the controlled and supportive environment of a rehabilitation facility and expected to continue exercising at home [Citation6–8].
One of the most common perceived barriers to adherence to rehabilitation exercises and physical activity in stroke patients is a lack of motivation [Citation9]. Motivation can be generally explained as a fundamental “drive” towards change. According to Deci and Ryan’s Self Determination Theory (SDT), motivation has three underlying Basic Psychosocial Needs (BPNs): Autonomy, Competence and Relatedness (10). The need for autonomy refers to the belief in being agentic and volitional in your actions, and it implies self-initiation and a choice over your behaviour. The need for competence refers to feeling capable and effective in the physical and social environment. The need for relatedness refers to connecting to a community or group and having a secure relational base [Citation10]. These three BPNs are critical resources for psychological well-being and autonomous, self-determined motivation and satisfying them increases motivation to pursue desired behaviour [Citation10]. Literature shows that higher motivation levels positively correlate with higher adherence to a rehabilitation program [Citation11,Citation12] and better rehabilitation outcomes [Citation3,Citation4,Citation13].
Various motivational strategies targeting at least one of the three BPNs are used in face-to-face sessions between therapists and patients [Citation14,Citation15]. Examples of this are the application of patients’ preferences (increasing autonomy), control of task difficulty (increasing competence) and involvement of a family member (increasing relatedness) [Citation14,Citation15]. During home-based rehabilitation, patients’ motivational needs are expected to differ from inpatient rehabilitation since there is no direct supervision, less face-to-face contact between patients and therapists and more distraction from the physical and social environment. Besides, stroke often affects the social relationships and roles of the patient in their home environment, for example, family members may become informal caregivers, and patients are less involved in community activities [Citation16,Citation17].
Recently, a Delphi study was conducted to identify strategies to facilitate adherence to home-based exercises after a stroke [Citation18]. Multiple strategies were classified, including using eHealth applications to prescribe exercises, provide feedback and motivate patients to follow home-based rehabilitation. However, the expert panel in this study did not include stroke survivors, and the study does not provide insights into patients’ needs [Citation18]. Since many eHealth applications face a rapid decrease in use and low adherence in the home environment [Citation19], a better understanding of their motivational needs regarding using home-based rehabilitation eHealth tools is needed.
To increase the understanding of motivation in stroke patients, evaluating the BPNs in combination with patient-specific contextual and personal factors that influence motivation is important. Multiple studies documented motivation as the interplay of individual characteristics, including individual factors such as personality, psychological attributes and health status, and contextual factors, which are the social context and the culturally enforced norms [Citation11,Citation14,Citation20,Citation21]. BPNs, as described by Deci and Ryan, are generic and do not specifically take into account these personal and contextual factors. Moreover, the feeling of autonomy, competence, and social roles and relationships change due to a chronic disabling condition, such as stroke [Citation22]. Thus, a specific patient population’s characteristics and home environment should not be neglected when providing or developing home-based rehabilitation.
This study investigates stroke patients’ motivation for home-based UE rehabilitation using eHealth tools and how this is embedded in the three BPNs. To account for the specific personal and contextual factors, we choose a qualitative study design, allowing us to acquire rich personal and contextual data conducted within a representative group of stroke patients at their own homes. With this knowledge, we expect to better align eHealth tools to the patient’s needs, resulting in increased therapy adherence. […]

Figure 1. The interactive demonstrator “EDO” and the three exercises included in the interface: (a) cylinder grasp, (b) pinch grasp, and (c) lifting and tilting movement.
A three-panel figure shows a tablet, a cylindrical-shaped object, and the arm and hand of a person performing three different exercises. In panel a), the person’s hand holds the object with a cylindrical grasp, and the object turns orange. Panel b) shows the person’s hand performing a pinch grip on top of the cylindrical object while the object turns green. In panel c), the person’s hand holds the object in a horizontal position without ground support, while the object turns green.
[WEB] Internet or In-Person PT?
Posted by Kostas Pantremenos in REHABILITATION, Tele/Home Rehabilitation on February 15, 2024

As more clinics begin opening their doors to patients after relying on remote options, it’s worth considering whether one is better than the other.
By Therese Casey, PT, DPT, and Gerald Dolce, PT, DPT, Cert-DRN, COMT, OCS
Telehealth’s Impact on Healthcare and Physical Therapy
Telemedicine, aka telehealth, has been rapidly growing over the past 5 years. Due to the COVID-19 pandemic, we have seen even more of an increase in demand for this service. While the pandemic is starting to slow, telehealth should remain an option for patients, as it accommodates travel and busy schedules. Rapidly evolving telemedicine has been able to provide increased access to high-quality healthcare and is cost-effective.
Further Reading: Overcoming Telehealth Barriers for Stroke Survivors
According to the Centers for Medicare and Medicaid Services (CMS), telemedicine is “a service that seeks to improve a patient’s health by permitting two-way, real-time interactive communication between the patient and the physician at a distant site.”¹ The concern in the physical therapy field remains: Which is better, telemedicine or in-clinic care? In order to answer this question, we have to dive into different diagnoses as well as visit how it may impact the care performed at home and in the clinic.
Benefits of In-Person Treatment
For certain conditions, in-person treatment has invaluable effects. The three major benefits of in-person treatment include manual therapy techniques, patient education, and ensuring proper body mechanics.
Manual Therapy
Manual therapy can consist of joint mobilizations, soft tissue mobilization, massage, scar mobilization, trigger point dry needling, Graston Technique, cupping, and more. These techniques require the skills of a licensed healthcare professional to be effective and safely completed. This is arguably the most skilled part of physical therapy and can make a significant impact on a patient’s pain management and recovery.
For example, a patient may require ankle joint mobilizations to improve motion needed to go down stairs. An individual with chronic headaches would likely benefit from a suboccipital release or dry needling to cervical muscles. Or a PT completing the Epley’s maneuver can fully resolve problems with dizziness for a patient who suffers from vertigo. These are techniques only a professional can perform and, ultimately, the power of touch helps to build rapport and trust with a patient.
Patient Education
For patients to fully understand their condition and appreciate the relevance of their treatment, education is key. Whether using a spine model, Netter Anatomy book, or layman’s terms, it is a PT’s role to convey the source of the condition and provide guidance on how to effectively manage it. For instance, a patient may not understand how pain in their back is causing numbness or tingling down their leg, or why weakness in their hips is causing pain on their outer knee.
After explaining how these items are interconnected, it is also necessary to ensure the patient understands why they are doing the therapeutic exercises that have been advised. For example, a PT can explain that they are performing sciatic nerve glides to decrease neural tension and subsequent tingling, or performing glute strengthening to prevent hip drop and subsequent knee pain while jogging.
Education can also be provided on self-mobilization techniques and utilization of therapy equipment to promote carryover between sessions and maintenance of therapeutic gains upon discharge. Patient education should start on day one and continue through the entirety of the plan of care.
Body Mechanics
Proper body mechanics are crucial to reduce stress on affected body regions and prevent reinjury. Squat form, gait mechanics on treadmill, and overhead lifts without compensation are examples of functional activities that require a skilled eye. Various views of squat, slow-motion video assessment on treadmill, and tactile cues to reduce excess upper trap activation during lifting help to ensure safe completion of these activities. Demonstration and feedback by a PT allow for improved comprehension of form by a patient.
While it could be argued that aspects of in-person treatment could be accomplished via telehealth, it would not be to the same degree. Personal touch and human interaction act as major drivers to patient success.
In Clinic vs At Home
As we look to expand how telehealth can contribute as a player in the future of healthcare, there are certain diagnoses that may be more advantageous to treat at home versus in the clinic. For example, much of low back research suggests that helping patients participate in functional movement helps improve symptoms over a set period of time, which could be completed at home. On the other hand, postoperative total hip arthroplasty (THA) may be better suited for in-clinic treatment. In the clinic, not only can a physical therapist provide guidance for maintaining hip precautions, but the postoperative THA patient also has access to the manual techniques the PT can perform and to certain equipment that may not be as readily available at home.
Further Reading: Revised Guidelines Regarding Cognitive Rehabilitation After TBI Focus on Telehealth
Whether in the clinic or at home, telehealth physical therapy clearly provides benefits and positive outcomes. It may be able to provide long-term relief and improvement of function for patients with various conditions. For example, in a trial of patients managed with total knee arthroplasty (TKA), a virtual PT program with skilled telerehabilitation was determined to be as effective as traditional PT for addressing function and disability and as safe as traditional PT in terms of pain and rehospitalization.² These findings suggest that virtual PT with a telehealth therapist for remote clinical monitoring and guidance should be considered for patients after TKA.² Overall, early intervention, consistency in treatment, and patient engagement will set up a patient for a successful outcome, regardless of the setting.
Equipment for In-Clinic and At Home
At-home equipment can have multiple advantages. First off, a patient can achieve similar results after successfully going through a demonstration in the clinic.
Traction
For example, a patient who is suffering from either cervical or lumbar discogenic pain may be able to obtain home lumbar and cervical traction units. In the clinic, we can utilize equipment such as the Chattanooga TX traction unit from Chattanooga Medical Supply, Chattanooga, Tenn, which can help with decompression, guided by the recommended parameters from the therapist. Fortunately, we can also achieve these same results with units such as ComforTrac CT by Zynex Medical, Englewood, Colo, which is more affordable and available for purchase by the patient. These units may also be covered by insurance, which makes them a more feasible option to continue treatment virtually once proper direction is given by the licensed clinician.
Strength and Conditioning
There are other conditions for which strength and conditioning equipment would be more appropriate. ACL reconstruction, ankle sprains, femoral acetabular impingement, and shoulder impingement are just a handful of conditions that would benefit from a structured program of progressive loading. An example would be increasing strength production after ACL reconstruction. After the initial stages of mini squat, progressing into 90-90 squatting at home with utilization of resistance bands can help make these exercises more challenging for the individual. Specifically, Cando Resistance bands made by Chattanooga Medical Supply can provide resistance to the lower extremities by stepping on the bands, creating a downward resistance effectively working lower extremity musculature.
Large
In-clinic equipment can be expensive and large, making it difficult to have at home. Something such as a simple leg press machine would not only suffice in strength production, but also to help improve knee range of motion for those progressing through ACL reconstruction, THA, and TKA. Having this type of equipment at the clinic is a benefit of attending in-person therapy sessions. The Cybex VR3 Leg Press made by Cybex International, Medway, Mass, is an example of a safe and effective machine that can achieve this in the clinic.
Small
We can also look to smaller equipment that can achieve balance and proprioception goals with this same patient population. The Fitterfirst Rockerboard from Fitterfirst in Calgary, Alberta, Canada, is a piece of equipment that we use in the clinic for this purpose. This is known as a professional device and would not be available to the general population.
Balance is a key ingredient in the treatment plan of patients with various conditions. Specifically, for patients who have a postoperative status, it may be beneficial for them to have a therapist to guard movements when using the Rockerboard as this may be unsafe for a patient to independently perform within their home, even if they did have access to one there. But the lack of availability of certain necessary equipment in patients’ homes is another example of how in-clinic treatment can provide benefits over telehealth.
Transitioning
As we look to achieve the best possible outcomes for our patients, we can select the best pieces of equipment to enhance recovery and advance patients in their treatment program. Once we achieve desired goals utilizing manual therapy, in-clinic equipment with therapist direction, and patient education, we can direct the patient to start to perform similar activities at home. Allowing patients to become independent and assisting them to acquire pieces of equipment that promote established goals set on the initial evaluation date will promote self-efficacy.
A Matter of Choice
To answer the question, “Is telehealth or in-clinic physical therapy superior?” we argue that both have an appropriate place in the healthcare field, but it ultimately depends on a patient’s condition and their access to technology as well as therapeutic equipment. A patient should select whichever treatment method allows them to feel most comfortable while at the same time maximizes their outcomes. PTP
Therese Casey, PT, DPT, is a physical therapist and clinic director at ATI Physical Therapy in Chicago. She received her Doctorate in Physical Therapy from Marquette University in 2017 and has been practicing as a PT at ATI for 4 years.
Gerald Dolce, PT, DPT, Cert-DRN, COMT, OCS, is a physical therapist and clinic director at ATI Physical Therapy in Las Vegas, specializing in advanced orthopedic conditions. A graduate from the Touro University Nevada physical therapy program, Dolce has been a practicing PT since 2017. For more information, contact PTPEditor@medqor.com.
References
- Kichloo A, Albosta M, Dettloff K, et al. Telemedicine, the current COVID-19 pandemic and the future: a narrative review and perspectives moving forward in the USA. Fam Med Community Health. 2020;8(3):e000530 doi: 10.1136/fmch-2020-000530
- Prvu Bettger J, Green CL, Holmes DN, et al. Effects of virtual exercise rehabilitation in-home therapy compare with traditional care after total knee arthroplasty. J Bone Joint Surg. 2020;102(2):101-109.
[Abstract + References] Combined noninvasive brain stimulation virtual reality for upper limb rehabilitation poststroke: A systematic review of randomized controlled trials
Posted by Kostas Pantremenos in Paretic Hand, REHABILITATION, Virtual reality rehabilitation on February 1, 2024
Abstract
Upper limb impairments are common consequences of stroke. Noninvasive brain stimulation (NIBS) and virtual reality (VR) play crucial roles in improving upper limb function poststroke. This review aims to evaluate the effects of combined NIBS and VR interventions on upper limb function post-stroke and to provide recommendations for future studies in the rehabilitation field. PubMed, MEDLINE, PEDro, SCOPUS, REHABDATA, EMBASE, and Web of Science were searched from inception to November 2023. Randomized controlled trials (RCTs) encompassed patients with a confirmed stroke diagnosis, administrated combined NIBS and VR compared with passive (i.e., rest) or active (conventional therapy), and included at least one outcome assessing upper limb function (i.e., strength, spasticity, function) were selected. The quality of the included studies was assessed using the Cochrane Collaboration tool. Seven studies met the eligibility criteria. In total, 303 stroke survivors (Mean age: 61.74 years) were included in this review. According to the Cochrane Collaboration tool, five studies were classified as “high quality,” while two were categorized as “moderate quality”. There are mixed findings for the effects of combined NIBS and VR on upper limb function in stroke survivors. The evidence for the effects of combined transcranial direct current stimulation and VR on upper limb function post-stroke is promising. However, the evidence regarding the effects of combined repetitive transcranial magnetic stimulation and VR on upper limb function is limited. Further randomized controlled trials with long-term follow-up are strongly warranted.
References
- Mortality and global health estimates (2019) Who.int. https://www.who.int/data/gho/data/themes/topics/topicdetails/GHO/gho-ghe-mortality-and-global-health-estimates
- Buma FE, Kwakkel G, Ramsey NF (2013) Understanding upper limb recovery after stroke. Restor Neurol Neurosci 31(6):707–722. https://doi.org/10.3233/rnn-130332Article PubMed Google Scholar
- Miller EL, Murray L, Richards L, Zorowitz RD, Bakas T, Clark PC, Billinger SA (2010) Comprehensive Overview of Nursing and interdisciplinary Rehabilitation care of the stroke patient. Stroke 41(10):2402–2448. https://doi.org/10.1161/str.0b013e3181e7512bArticle PubMed Google Scholar
- Kwakkel G, Kollen BJ (2012) Predicting Activities after Stroke: What is Clinically Relevant? Int J Stroke 8(1):25–32. https://doi.org/10.1111/j.1747-4949.2012.00967.xArticle Google Scholar
- Pollock A, Farmer SE, Brady M, Langhorne P, Mead G, Mehrholz J, Van Wijck F (2014) Interventions for improving upper limb function after stroke. Cochrane Libr. https://doi.org/10.1002/14651858.cd010820.pub2Article Google Scholar
- Cassidy JM, Cramer SC (2016) Spontaneous and Therapeutic-Induced Mechanisms of functional recovery after Stroke. Transl Stroke Res 8(1):33–46. https://doi.org/10.1007/s12975-016-0467-5Article PubMed PubMed Central CAS Google Scholar
- Warraich Z, Kleim JA (2010) Neural plasticity: the biological substrate for neurorehabilitation. PM&R 2(12S). https://doi.org/10.1016/j.pmrj.2010.10.016
- Kleim JA, Jones TA (2008) Principles of Experience-Dependent Neural Plasticity: Implications for rehabilitation after Brain damage. Journal of Speech Language and Hearing Research 51(1). https://doi.org/10.1044/1092-4388(2008/018
- Subramanian S, Lourenço CB, Chilingaryan G, Sveistrup H, Levin MF (2012) Arm motor recovery using a virtual reality intervention in chronic stroke. Neurorehabil Neural Repair 27(1):13–23. https://doi.org/10.1177/1545968312449695Article PubMed Google Scholar
- Alashram AR, Padua E, Aburub A, Raju M, Annino G (2022) Transcranial direct current stimulation for upper extremity spasticity rehabilitation in stroke survivors: A systematic review of randomized controlled trials. PM&R 15(2):222–234. https://doi.org/10.1002/pmrj.12804Article Google Scholar
- Sherman WR, Craig AB (2003) Understanding Virtual Reality—Interface, application, and design. Presence: Teleoperators Virtual Environ 12(4):441–442Article Google Scholar
- Sveistrup H (2004) Motor rehabilitation using virtual reality. J Neuroeng Rehabil 1(1):1–10. https://doi.org/10.1186/1743-0003-1-10Article Google Scholar
- Saleh S, Fluet GG, Qiu Q, Merians AS, Adamovich SV, Tunik E (2017) Neural Patterns of Reorganization after Intensive Robot-Assisted Virtual Reality Therapy and Repetitive Task Practice in Patients with Chronic Stroke. Frontiers in Neurology 8. https://doi.org/10.3389/fneur.2017.00452
- Alashram AR, Annino G, Padua E, Romagnoli C, Mercuri NB (2019) Cognitive rehabilitation post traumatic brain injury: A systematic review for emerging use of virtual reality technology. J Clin Neurosci 66:209–219. https://doi.org/10.1016/j.jocn.2019.04.026Article PubMed Google Scholar
- Chi B, Chau B, Yeo E, Ta P (2019) Virtual reality for spinal cord injury-associated neuropathic pain: Systematic review. Ann Phys Rehabil Med 62(1):49–57. https://doi.org/10.1016/j.rehab.2018.09.006Article PubMed CAS Google Scholar
- Alashram AR, Padua E, Annino G (2022) Virtual reality for balance and mobility rehabilitation following traumatic brain injury: A systematic review of randomized controlled trials. J Clin Neurosci 105:115–121. https://doi.org/10.1016/j.jocn.2022.09.012Article PubMed Google Scholar
- Alashram AR, Padua E, Hammash AK, Lombardo M, Annino G (2020) Effectiveness of virtual reality on balance ability in individuals with incomplete spinal cord injury: A systematic review. J Clin Neurosci 72:322–327. https://doi.org/10.1016/j.jocn.2020.01.037Article PubMed Google Scholar
- Teasell R, Mehta S, Pereira S, McIntyre A, Janzen S, Allen L, Lobo L, Viana R (2012) Time to rethink Long-Term rehabilitation management of stroke patients. Top Stroke Rehabil 19(6):457–462. https://doi.org/10.1310/tsr1906-457Article PubMed Google Scholar
- Alashram AR, Padua E, Annino G (2022) Noninvasive brain stimulation for cognitive rehabilitation following traumatic brain injury: a systematic review. Appl Neuropsychol Adult 30(6):814–829. https://doi.org/10.1080/23279095.2022.2091440Article PubMed Google Scholar
- Alashram AR, Padua E, Romagnoli C, Raju M, Annino G (2021) Effects of Repetitive transcranial magnetic stimulation on Upper extremity spasticity Post-Stroke: A Systematic review. Physikalische Medizin Rehabilitationsmedizin Kurortmedizin. https://doi.org/10.1055/a-1691-9641Article Google Scholar
- Schlemm E, Schulz R, Bönstrup M, Krawinkel L, Fiehler J, Gerloff C, Thomalla G, Cheng B (2020) Structural brain networks and functional motor outcome after stroke—a prospective cohort study. Brain Communications 2(1). https://doi.org/10.1093/braincomms/fcaa001
- Hara T, Shanmugalingam A, McIntyre A, Burhan AM (2021) The Effect of Non-Invasive Brain Stimulation (NIBS) on attention and Memory Function in Stroke Rehabilitation Patients: A Systematic Review and Meta-Analysis. Diagnostics 11(2):227. https://doi.org/10.3390/diagnostics11020227Article PubMed PubMed Central Google Scholar
- Dhaliwal SK, Meek BP, Modirrousta M (2015) Non-Invasive brain stimulation for the treatment of symptoms following traumatic brain injury. Frontiers in Psychiatry 6. https://doi.org/10.3389/fpsyt.2015.00119
- Rossi S, Hallett M, Rossini PM, Pascual-Leone Á (2009) Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol 120(12):2008–2039. https://doi.org/10.1016/j.clinph.2009.08.016Article PubMed PubMed Central Google Scholar
- Tassinari CA, Cincotta M, Zaccara G, Michelucci R (2003) Transcranial magnetic stimulation and epilepsy. Clin Neurophysiol 114(5):777–798. https://doi.org/10.1016/s1388-2457(03)00004-xArticle PubMed Google Scholar
- Sandrini M, Umiltà C, Rusconi E (2011) The use of transcranial magnetic stimulation in cognitive neuroscience: A new synthesis of methodological issues. Neurosci Biobehav Rev 35(3):516–536. https://doi.org/10.1016/j.neubiorev.2010.06.005Article PubMed Google Scholar
- Peinemann A, Reimer B, Löer C, Quartarone A, Münchau A, Conrad B, Siebner HR (2004) Long-lasting increase in corticospinal excitability after 1800 pulses of subthreshold 5 Hz repetitive TMS to the primary motor cortex. Clin Neurophysiol 115(7):1519–1526. https://doi.org/10.1016/j.clinph.2004.02.005Article PubMed Google Scholar
- Mansur CG, Fregni F, Boggio PS, Riberto M, Neto JG, Santos CMD, Wagner T, Rigonatti SP, Marcolin MA, Pascual-Leone A (2005) A sham stimulation-controlled trial of rTMS of the unaffected hemisphere in stroke patients. Neurology 64(10):1802–1804. https://doi.org/10.1212/01.wnl.0000161839.38079.92Article PubMed CAS Google Scholar
- Huang Y, Edwards MJ, Rounis E, Rothwell JC (2007) Theta burst stimulation on human motor cortex. Clin Neurophysiol 118(5):e151. https://doi.org/10.1016/j.clinph.2006.07.224Article Google Scholar
- Schicktanz N, Fastenrath M, Milnik A, Spalek K, Auschra B, Nyffeler T, Papassotiropoulos A, De Quervain DJ, Schwegler K (2015) Continuous Theta Burst Stimulation over the Left Dorsolateral Prefrontal Cortex Decreases Medium Load Working Memory Performance in Healthy Humans. PLOS ONE 10(3):e0120640. https://doi.org/10.1371/journal.pone.0120640Article PubMed PubMed Central CAS Google Scholar
- Oberman LM, Edwards D, Eldaief MC, Pascual-Leone Á (2011) Safety of theta burst Transcranial Magnetic Stimulation: A Systematic Review of the literature. J Clin Neurophysiol 28(1):67–74. https://doi.org/10.1097/wnp.0b013e318205135fArticle PubMed PubMed Central Google Scholar
- George MS, Nahas Z, Borckardt JJ, Anderson B, Foust MJ, Burns C, Köse S, Short EB (2007) Brain stimulation for the treatment of psychiatric disorders. Curr Opin Psychiatry 20(3):250–254. https://doi.org/10.1097/yco.0b013e3280ad4698Article PubMed Google Scholar
- Wassermann EM, Lisanby SH (2001) Therapeutic application of repetitive transcranial magnetic stimulation: a review. Clin Neurophysiol 112(8):1367–1377. https://doi.org/10.1016/s1388-2457(01)00585-5Article PubMed CAS Google Scholar
- Zaghi S, Acar M, Hultgren BA, Boggio PS, Fregni F (2009) Noninvasive Brain Stimulation with Low-Intensity Electrical Currents: Putative Mechanisms of Action for Direct and Alternating Current Stimulation. Neuroscientist 16(3):285–307. https://doi.org/10.1177/1073858409336227Article PubMed Google Scholar
- Williams J, Imamura M, Fregni F (2009) Updates on the use of non-invasive brain stimulation in physical and rehabilitation medicine. J Rehabil Med 41(5):305–311. https://doi.org/10.2340/16501977-0356Article PubMed Google Scholar
- Cassani R, Novak GS, Falk TH, De Oliveira AA (2020) Virtual reality and non-invasive brain stimulation for rehabilitation applications: a systematic review. Journal of Neuroengineering and Rehabilitation 17(1). https://doi.org/10.1186/s12984-020-00780-5
- Massetti T, Crocetta TB, Da Silva TD, Trevizan IL, Arab C, Caromano FA, De Mello Monteiro CB (2016) Application and outcomes of therapy combining transcranial direct current stimulation and virtual reality: a systematic review. Disabil Rehabil Assist Technol 12(6):551–559. https://doi.org/10.1080/17483107.2016.1230152Article PubMed Google Scholar
- Subramanian S, Prasanna SS (2018) Virtual reality and noninvasive brain stimulation in stroke: How effective is their combination for Upper limb motor Improvement?—A Meta-Analysis. PM&R 10(11):1261–1270. https://doi.org/10.1016/j.pmrj.2018.10.001Article Google Scholar
- Meng J, Yan Z, Gu F, Tao X, Xue T, Liŭ D, Wang Z (2023) Transcranial direct current stimulation with virtual reality versus virtual reality alone for upper extremity rehabilitation in stroke: A meta-analysis. Heliyon 9(1):e12695. https://doi.org/10.1016/j.heliyon.2022.e12695Article PubMed Google Scholar
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann T, Mulrow CD, Shamseer L, Tetzlaff J, Akl EA, Brennan S, Chou R, Glanville J, Grimshaw J, Hróbjartsson A, Lalu MM, Li T, Loder E, Mayo‐Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch V, Whiting P, Moher D (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ :n71. https://doi.org/10.1136/bmj.n71
- Liberati A, Altman DG, Tetzlaff J, Mulrow CD, Gøtzsche PC, Ioannidis JPA, Clarke M, Devereaux PJ, Kleijnen J, Moher D (2009) The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies that Evaluate Health Care Interventions: Explanation and Elaboration. PLoS Med 6(7):e1000100. https://doi.org/10.1371/journal.pmed.1000100Article PubMed PubMed Central Google Scholar
- Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savović J, Schulz KF, Weeks L, Sterne JA (2011) The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343(oct18 2):d5928. https://doi.org/10.1136/bmj.d5928Article PubMed PubMed Central Google Scholar
- Jørgensen L, Paludan-Müller AS, Laursen DRT, Savović J, Boutron I, Sterne J a C, Higgins JPT, Hróbjartsson A (2016) Evaluation of the Cochrane tool for assessing risk of bias in randomized clinical trials: overview of published comments and analysis of user practice in Cochrane and non-Cochrane reviews. Systematic Reviews 5(1). https://doi.org/10.1186/s13643-016-0259-8
- Yao X, Cui L, Wang J, Feng W, Bao Y, Xie Q (2020) Effects of transcranial direct current stimulation with virtual reality on upper limb function in patients with ischemic stroke: a randomized controlled trial. Journal of Neuroengineering and Rehabilitation 17(1). https://doi.org/10.1186/s12984-020-00699-x
- Lee SJ, Chun MH (2014) Combination transcranial direct current stimulation and virtual reality therapy for upper extremity training in patients with subacute stroke. Arch Phys Med Rehabil 95(3):431–438. https://doi.org/10.1016/j.apmr.2013.10.027Article PubMed Google Scholar
- Lee S, Cha H-G (2021) The effect of clinical application of transcranial direct current stimulation combined with non-immersive virtual reality rehabilitation in stroke patients. Technol Health Care 30(1):117–127. https://doi.org/10.3233/thc-212991Article Google Scholar
- Llorens RC, Fuentes MA, Llorens RC, Latorre J, Alcañíz M, Colomer C, Noé E (2021) Effectiveness of a combined transcranial direct current stimulation and virtual reality-based intervention on upper limb function in chronic individuals post-stroke with persistent severe hemiparesis: a randomized controlled trial. Journal of Neuroengineering and Rehabilitation 18(1). https://doi.org/10.1186/s12984-021-00896-2
- Viana R, Laurentino GEC, De Souza RMCR, Fonseca JB, Filho EM, Dias S, Teixeira-Salmela LF, Monte-Silva K (2014) Effects of the addition of transcranial direct current stimulation to virtual reality therapy after stroke: A pilot randomized controlled trial. NeuroRehabilitation 34(3):437–446. https://doi.org/10.3233/nre-141065Article PubMed CAS Google Scholar
- Cheng Z, Liao W, Xia W (2015) Effect of combined low-frequency repetitive transcranial magnetic stimulation and virtual reality training on upper limb function in subacute stroke: a double-blind randomized controlled trail. J Huazhong Univ Sci Technol 35(2):248–254. https://doi.org/10.1007/s11596-015-1419-0Article Google Scholar
- Chen Y, Chen C-L, Huang Y, Chen H-C, Chen C-Y, Wu C, Lin K (2021) Augmented efficacy of intermittent theta burst stimulation on the virtual reality-based cycling training for upper limb function in patients with stroke: a double-blinded, randomized controlled trial. Journal of Neuroengineering and Rehabilitation 18(1). https://doi.org/10.1186/s12984-021-00885-5
- Oosterveer DM, Wermer MJH, Volker G, Vlieland TPMV (2022) Are there differences in Long-Term functioning and recovery between hemorrhagic and ischemic stroke patients receiving rehabilitation? J Stroke Cerebrovasc Dis 31(3):106294. https://doi.org/10.1016/j.jstrokecerebrovasdis.2021.106294Article PubMed Google Scholar
- Perna R, Temple J (2015) Rehabilitation Outcomes: Ischemic versus Hemorrhagic Strokes. Behav Neurol 2015:1–6. https://doi.org/10.1155/2015/891651Article Google Scholar
- Winstein CJ, Stein J, Arena R, Bates B, Cherney LR, Cramer SC,…Zorowitz RD (2016) Guidelines for Adult Stroke Rehabilitation and Recovery. Stroke, 47(6). https://doi.org/10.1161/str.0000000000000098
- Bernhardt J, Godecke E, Johnson L, Langhorne P (2017) Early rehabilitation after stroke. Curr Opin Neurol 30(1):48–54. https://doi.org/10.1097/wco.0000000000000404Article PubMed Google Scholar
- Kwakkel G, Kollen BJ, Van Der Grond JV, Prevo AJH (2003) Probability of regaining dexterity in the flaccid upper limb. Stroke 34(9):2181–2186. https://doi.org/10.1161/01.str.0000087172.16305.cdArticle PubMed Google Scholar
- Nishimura Y, Onoe H, Morichika Y, Perfiliev S, Tsukada H, Isa T (2007) Time-Dependent central compensatory mechanisms of finger dexterity after spinal cord injury. Science 318(5853):1150–1155. https://doi.org/10.1126/science.1147243Article PubMed CAS Google Scholar
- Dhamoon MS, Moon Y, Paik MC, Boden-Albala B, Rundek T, Sacco RL, Elkind MSV (2009) Long-Term functional recovery after first ischemic stroke. Stroke 40(8):2805–2811. https://doi.org/10.1161/strokeaha.109.549576Article PubMed PubMed Central Google Scholar
- Kolmos M, Christoffersen LC, Kruuse C (2021) Recurrent Ischemic Stroke – A Systematic Review and Meta-Analysis. J Stroke Cerebrovasc Dis 30(8):105935. https://doi.org/10.1016/j.jstrokecerebrovasdis.2021.105935Article PubMed Google Scholar
- D’Souza CE, Greenway MRF, Graff-Radford J, Meschia JF (2021) Cognitive Impairment in Patients with Stroke. Semin Neurol 41(01):075–084. https://doi.org/10.1055/s-0040-1722217Article Google Scholar
- De Luca R, Russo M, Naro A, Tomasello P, Leonardi S, Santamaria F, Desireè L, Bramanti A, Silvestri G, Bramanti P, Calabrò RS (2018) Effects of virtual reality-based training with BTs-Nirvana on functional recovery in stroke patients: preliminary considerations. Int J Neurosci 128(9):791–796. https://doi.org/10.1080/00207454.2017.1403915Article PubMed Google Scholar
- Zhang Q, Fu Y, Lu Y, Zhang Y, Huang Q, Yang Y, Zhang K, Li M (2021) Impact of Virtual Reality-Based Therapies on Cognition and Mental Health of Stroke Patients: Systematic Review and Meta-analysis. J Med Internet Res 23(11):e31007. https://doi.org/10.2196/31007Article PubMed PubMed Central Google Scholar
- Wu J, Zeng A, Chen Z, Wei Y, Huang K, Chen J, Ren Z (2021) Effects of Virtual Reality Training on Upper Limb Function and Balance in Stroke Patients: Systematic Review and Meta-Meta-Analysis. J Med Internet Res 23(10):e31051. https://doi.org/10.2196/31051Article PubMed PubMed Central Google Scholar
- Tedla JS, Sangadala DR, Reddy RS, Gular K, Kakaraparthi VN, Asiri F (2023) Transcranial direct current stimulation (tDCS) effects on upper limb motor function in stroke: an overview review of the systematic reviews. Brain Inj 37(2):122–133. https://doi.org/10.1080/02699052.2022.2163289Article PubMed Google Scholar
- Alashram AR, Padua E, Romagnoli C, Annino G (2019) Effectiveness of focal muscle vibration on hemiplegic upper extremity spasticity in individuals with stroke: A systematic review. NeuroRehabilitation 45(4):471–481. https://doi.org/10.3233/nre-192863Article PubMed Google Scholar
- Annino G, Alashram AR, Alghwiri AA, Romagnoli C, Messina G, Tancredi V, Padua E, Mercuri NB (2019) Effect of segmental muscle vibration on upper extremity functional ability poststroke. Medicine 98(7):e14444. https://doi.org/10.1097/md.0000000000014444Article PubMed PubMed Central Google Scholar
- Alashram AR, Annino G, Al-Qtaishat M, Padua E (2020) Mental practice combined with physical practice to enhance upper extremity functional ability poststroke: A Systematic review. J Stroke Med 3(2):51–61. https://doi.org/10.1177/2516608520943793Article Google Scholar
- Alashram AR, Annino G, Mercuri NB (2019) Task-oriented motor learning in upper extremity rehabilitation post stroke. J Stroke Med 2(2):95–104. https://doi.org/10.1177/2516608519864760Article Google Scholar
- Martin S, Cordeiro L, Richardson P, Davis S, Tartaglia N (2018) The association of motor skills and adaptive functioning in XXY/Klinefelter and XXYY syndromes. Phys Occup Ther Pediatr 39(4):446–459. https://doi.org/10.1080/01942638.2018.1541040Article PubMed PubMed Central Google Scholar
- Johnson D, Harris JE, Stratford PW, Richardson J (2018) Interrater reliability of three versions of the Chedoke arm and hand activity inventory. Physiother Can 70(2):133–140. https://doi.org/10.3138/ptc.2016-70Article PubMed PubMed Central Google Scholar
- Uswatte G, Taub E, Morris D, Vignolo MJ, McCulloch K (2005) Reliability and validity of the Upper-Extremity Motor Activity Log-14 for measuring Real-World arm use. Stroke 36(11):2493–2496. https://doi.org/10.1161/01.str.0000185928.90848.2eArticle PubMed Google Scholar
- Egger M, Smith GD (1998) meta-analysis bias in location and selection of studies. BMJ 316(7124):61–66. https://doi.org/10.1136/bmj.316.7124.61Article PubMed PubMed Central CAS Google Scholar
- Higgins JPT, Green S (2008) Cochrane Handbook for Systematic Reviews of Interventions. In: Wiley eBooks
[Abstract] Exergames as a rehabilitation tool to enhance the upper limbs functionality and performance in chronic stroke survivors: a preliminary study
Posted by Kostas Pantremenos in Paretic Hand, REHABILITATION, Video Games/Exergames on January 30, 2024
Post-stroke hemiplegia commonly occurs in stroke survivors, negatively impacting the quality of life. Despite the benefits of initial specific post-acute treatments at the hospitals, motor functions and physical mobility need to be constantly stimulated to avoid regression and subsequent hospitalizations for further rehabilitation treatments. This preliminary study proposes using gamified tasks in a virtual environment to stimulate and maintain upper limb mobility through a single RGB-D camera-based vision system (using Microsoft Azure Kinect DK). This solution is suitable for easy deployment and use in home environments. A cohort of 10 post-stroke subjects attended a 2-week gaming protocol consisting of Lateral Weightlifting (LWL) and Frontal Weightlifting (FWL) gamified tasks and gait as the instrumental evaluation task. Despite its short duration, there were statistically significant results (p < 0.05) between the baseline (T0) and the end of the protocol (TF) for Berg Balance Scale and Time Up-and-Go (9.8% and -12.3%, respectively). LWL and FWL showed significant results for unilateral executions: rate in FWL had an overall improvement of 38.5% (p < 0.001) and 34.9% (p < 0.01) for the paretic and non-paretic arm, respectively; similarly, rate in LWL improved by 19.9% (p < 0.05) for the paretic arm and 29.9% (p < 0.01) for non-paretic arm. Instead, bilateral executions had significant results for rate and speed: considering FWL, there was an improvement in rate with p < 0.01 (31.7% for paretic arm and 37.4% for non-paretic arm), whereas speed improved by 31.2% (p < 0.05) and 41.7% (p < 0.001) for the paretic and non-paretic arm, respectively; likewise, LWL showed improvement in rate with p < 0.001 (29.% for paretic arm and 27.8% for non-paretic arm) and in speed with 23.6% (p < 0.05) and 23.5% (p < 0.01) for the paretic and non-paretic arms, respectively. No significant results were recorded for gait task, although an overall good improvement was detected for arm swing asymmetry (-22.6%). Hence, this study suggests the potential benefits of continuous stimulation of upper limb function through gamified exercises and performance monitoring over medium-long periods in the home environment, thus facilitating the patient’s general mobility in daily activities.
[Abstract + References] Effects of robotic-assisted gait training on physical capacity, and quality of life among chronic stroke patients: A randomized controlled study
Posted by Kostas Pantremenos in Gait Rehabilitation - Foot Drop, Rehabilitation robotics on January 24, 2024
Abstract
Background
Even though robotic therapy is becoming more commonly used in research protocols for lower limb stroke rehabilitation, there still is a significant gap between research evidence and its use in clinical practice. Therefore, the present study was designed assuming that the wearable mobile gait device training for chronic stroke patients might have different effects on functional independence when compared to training with a stationary gait device. The present study aims to examine the effects of gait training with ExoAthlet exoskeleton and Lokomat Free-D on functional independence, functional capacity, and quality of life in chronic stroke patients.
Methods
The present study included 32 chronic stroke patients. Participants were randomly divided into two groups. Functional independence of patients was evaluated by using Functional Independence Measure (FIM), physical function was assessed by using the 30-second chair stand test (30-CST), functional capacity was measured by using the 6-Minute Walk Test (6MWT), and quality of life was assessed by using Short Form 36 (SF36). All participants underwent a conventional physiotherapy program for eight weeks, three sessions per week, and each session lasted 60 min. After the physiotherapy program, one group received gait training by using ExoAthlet exoskeleton (ExoAtlet 1 model/2019, Russia), while the other group received training by using Lokomat Free-D (Hocoma, Lokomat Pro Free-D model/2015, Switzerland). Participants were assessed at baseline and post-intervention.
Results
Results achieved in this study revealed that there was a statistically significant difference between FIM, 30-CST, 6MWT, and SF36 scores before and after the treatment in both groups (p < 0.05).There was no difference in FIM, 30-CST, and 6MWT results between Exoskeleton ExoAthlet and Lokomat Free-D groups (p > 0.05). However, there was a statistically significant difference between Exoskeleton ExoAthlet and Lokomat Free-D groups in terms of SF-36 sub-parameters “vitality”, “mental health”, “bodily pain”, and “general health perception” (p < 0.05).
Conclusions
This study demonstrated that the use of ExoAthlet exoskeleton and Lokomat Free-D in addition to conventional physiotherapy, was effective in improving functional independence, physical function, functional capacity, and quality of life among chronic stroke patients. Incorporation of robotic gait aids into rehabilitation for chronic stroke patients might offer significant advantages.
Introduction
Stroke is defined as a condition that develops due to a disturbance in brain functions, either in a specific location or in the whole brain, rapidly manifests symptoms, and these symptoms persist for one day or longer, or result in death [1]. Motor functions are affected in 65 % of chronic stroke patients, and the majority of these patients experience a reduced level of functional independence [2]. It is believed that the most prominent challenges faced by chronic stroke patients are the distance walked in 6 min and the decrease in functional capacity [3]. In addition, cohort studies also reported that 22 % of chronic stroke patients did not regain any walking function [4].
A significant portion of chronic stroke patients suffer not only from physical disability but also from cognitive and emotional disorders [5]. General predictors of the poor quality of life after a stroke were reported to include medical comorbidities, loss of physical function, social role difficulties, emotional involvement, and depression [6], [7], [8].
In recent years, robotic technology has exhibited notable advancement thanks to faster and more powerful computers, innovative computational methodologies, and a broader range of electromechanical components. This technological advancement also made robotics suitable for rehabilitation interventions, and robotic rehabilitation is a promising method for treating patients with motor disorders. Its significance lies in its the potential to increase and carefully control the dosage of therapy [9], [10]. However, robotic rehabilitation does not solely focus on augmenting the quantity and intensity of treatment.
Robotic systems not only generate simple and repetitive stereotypical movement patterns but can also be utilized in order to provide patients with more intricate and controlled multisensory stimuli [11]. It can be seen that the effect of rehabilitation technology on functional outcomes can be optimized by affording the nervous system greater opportunities to experience genuine activity-related sensorimotor input [12]. Nevertheless, there are ongoing studies examining the therapeutic effectiveness of robotic rehabilitation. In clinical rehabilitation practices addressing chronic stroke patients, robotic technologies are employed for gait training, providing opportunities to move freely on a stationary or mobile basis. Robotic devices are utilized as assistive, rehabilitative, and augmentative instruments in lower extremity rehabilitation for neurological conditions [13]. Considering the lower extremity rehabilitation, the Lokomat, utilized as a stationary device, was demonstrated to be effective in improving walking quality, speed, and balance in conditions such as Multiple Sclerosis, Cerebral Palsy, Parkinson’s disease, Brown-Sequard syndrome, and vascular dementia [14]. Moreover, Lokomat was also reported to be an effective approach used in the rehabilitation of chronic stroke patients. In the literature, a retrospective case-control study examined the efficacy of Lokomat Free-D on functional independence, functional capacity, and balance in chronic stroke patients [15]. It was also reported that Lokomat Free-D is effective in chronic stroke individuals, not only affecting motor functions such as walking, balance, muscle strength, walking ability, and speed but also cognitive and emotional status. [14]. However, to the best of our knowledge, there is no study examining the effectiveness of Lokomat on the quality of life in chronic stroke individuals could be found. Another method utilized for lower extremity rehabilitation is the use of mobile gait devices. Devices such as MIRAD, XoR, and ExoAthlet exoskeleton are some of them [13]. The ExoAthlet exoskeleton was found to increase gait speed and stability, as well as reducing body sway, in conditions such as Multiple Sclerosis and spinal cord injuries [16], [17]. In a randomized controlled study comparing ExoAthlet exoskeleton and traditional physiotherapy in chronic stroke patients, the ExoAthlet exoskeleton group exhibited significant improvements when compared to the traditional physiotherapy group. The ExoAthlet exoskeleton group had a decrease in hemiparesis severity, an increase in paretic limb muscle strength, improvement in balance, and notable enhancements in the walking process and speed [18]. However, there is no study available that examines the effectiveness of the ExoAthlet exoskeleton on quality of life in chronic stroke patients. In both literature and clinical practice, there are gaps concerning the effectiveness of Lokomat Free-D and ExoAthlet exoskeleton on different parameters. There is no study available that has comparing these two different methods among chronic stroke patients. Finally, this study aims to examine the effects of gait training with the ExoAthlet exoskeleton and Lokomat Free-D on functional independence, functional capacity, and quality of life in chronic stroke patients, as well as to investigate whether there are any different effects.
Section snippets
Study design and ethics
Study design: Randomized Controlled Study.
Ethics: The study protocol was approved by from the institutional ethics board of Üsküdar University’s Non-Interventional Ethics Committee (Approval no = 61351342/February 2021-66/26.02.2021). The present study was carried out in accordance with the principles outlined in the Declaration of Helsinki. Participants, who voluntarily agreed to participate in this study, signed a written consent form. The paper is registered with ClinicalTrials.gov, and the
Results
Initially, 40 chronic stroke patients were involved in the study, and 8 individuals did not meet the inclusion criteria. Thus, the study was completed with a total of 32 individuals. The flow chart of the study is presented in Fig. 3.
The sociodemographic characteristics of the chronic stroke patients included in the study are shown in Table 1.
The results of this study showed that there was a statistically significant difference between functional independence, functional capacity, and quality
Discussion
Significant advancements have been achieved in robotic technology, especially in the last ten years, and the use of robotic technology in healthcare has increased. The use of robotic technology increased in post-stroke rehabilitation due to its advantages such as performing movements very similar to normal activity, providing continuous stimulation of the central nervous system, and creating treatment options with appropriate intensity and dosage for the patient during the rehabilitation
Limitations and future directions
The study has some limitations. The long-term effects of robotic-assisted gait training were not evaluated in this study. Additionally, different psychometric properties that would affect the quality of life were ignored. Future studies taking these parameters into consideration may provide different perspectives on the interpretation of results.
Conclusion
In conclusion, the results achieved in this study showed that gait training with Exoskeleton ExoAthlete and gait training with Lokomat Free-D for eight weeks administered in addition to conventional physiotherapy have positive effects on functional independence, functional capacity, and quality of life parameters in post-stroke patients. Training with Exoskeleton ExoAthlet had a more positive effect on the quality-of-life sub-parameters, such as vitality, mental health, bodily pain, and general
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References (45)
- S. Wist et al.Muscle strengthening for hemiparesis after stroke: A meta-analysisAnn Phys Rehabil Med(2016)
- H.S. Jørgensen et al.Recovery of walking function in stroke patients: the Copenhagen Stroke StudyArch Phys Med Rehabil(1995)
- R.J. NudoFunctional and structural plasticity in motor cortex: implications for stroke recoveryPhysical Medicine and Rehabilitation Clinics(2003)
- I. Schwartz et al.The effectiveness of locomotor therapy using robotic-assisted gait training in subacute stroke patients: a randomized controlled trialPMR(2009)
- GBD. Stroke collaborators. global, regional, and national burden of stroke, 1990-2016: a systematic analysis for the…
- Clara Selves, Gaëtan Stoquart, Thierry Lejeune. Gait rehabilitation after stroke: review of the evidence of predictors,…
- J.P.L. Slenders et al.Early cognitive and emotional outcome after stroke is independent of discharge destinationJ Neurol(2020)
- G.O. Vincent-Onabajo et al.Consistent determinants of health-related quality of life in the first 12 months after stroke: a prospective study in NigeriaTop Stroke Rehabil(2015)
- A.C. Jonsson et al.Determinants of quality of life in stroke survivors andtheir informal caregiversStroke(2005)
- Samsa GP, Matchar DB. How strong is the relationshipbetween functional status and quality of life among personswith…
- A. Esquenazi et al.Robotic-assisted gait training and restorationAm J Phys Med Rehabil(2012)
- L. PignoloRobotics in neuro-rehabilitationJ Rehabil Med(2009)
- A.B. KeelingUnderstanding Stroke Rehabilitation Progression in a Robotic Rehabilitation TrialCumming School of Medicine(2020)
- H. Lee et al.Lower limb exoskeleton systems—overviewWearable Robotics(2020)
- R.S. Calabrò et al.Robotic gait rehabilitation and substitution devices in neurological disorders: where are we now?Neurol Sci(2016)
- A. Manuli et al.Calabrò RS.J Is intensive gait training feasible and effective at old age? A retrospective case-control study on the use of Lokomat Free-D in patients with chronic strokeClin Neurosci(2021 Oct)
- S.V. Kotov et al.The efficacy of the exoskeleton ExoAtlet to restore walking in patients with multiple sclerosis. Zhurnal nevrologii i psikhiatrii imeni SSKorsakova(2017)
- E.Y. Shapkova et al.Exoskeleton walk training in paralyzed individuals benefits from transcutaneous lumbar cord tonic electrical stimulationFront Neurosci(2020)
- S.V. Kotov et al.Efficiency of leg exoskeleton use in rehabilitation of cerebral stroke patientsSerbian journal of experimental and clinical research(2021)
- R. Mustafaoglu et al.Does robot-assisted gait training improve mobility, activities of daily living and quality of life in stroke? A single-blinded, randomized controlled trialActa Neurol Belg(2020)
- A.A. Küçükdeveci et al.Adaptation of the Functional Independence Measure for use in TurkeyClin Rehabil(2001)
- Mcleod JC, Ward SJ, Hicks AL. Evaluation of the Keeogo™ Dermoskeleton. Disabil Rehabil Assist Technol. 2019…
There are more references available in the full text version of this article.
