Posts Tagged cognition
[Abstract + References] Effects of immersive and non-immersive virtual reality-based rehabilitation training on cognition, motor function, and daily functioning in patients with mild cognitive impairment or dementia: A systematic review and meta-analysis
Posted by Kostas Pantremenos in REHABILITATION, Virtual reality rehabilitation on November 29, 2023
Abstract
Objective
To examine the effectiveness of virtual reality (VR)-based rehabilitation training in improving cognition, motor function, and daily functioning in patients with mild cognitive impairment and dementia.
Data sources
A systematic review of published literature was conducted using PubMed, Web of Science, Elsevier, Embase, Cochrane, CNKI, Networked Digital Library of Theses and Dissertations.
Methods
The search period was from inception to 7 October 2023. Eligible studies were randomized controlled trials evaluating the efficacy of VR-based rehabilitation training in patients with mild cognitive impairment or dementia versus control subjects. Methodologic quality was assessed with the Cochrane risk of bias tool, and outcomes were calculated as the standard mean difference between participant groups with 95% confidence interval.
Results
A total of 21 randomized controlled trials with 1138 patients were included. The meta-analysis showed that VR-based rehabilitation training had significant effects on Montreal Cognitive Assessment (SMD: 0.50; 95%CI: 0.05 to 0.95; P = 0.030), Trail-making test A (SMD: −0.38; 95%CI: −0.61 to −0.14; P = 0.002), and Berg Balance Scale scores (SMD: 0.79; 95%CI: 0.13 to 1.45; P = 0.020). A subgroup analysis revealed that the type of VR, and duration and frequency of interventions had statistically significant effects on cognition and motor function.
Conclusion
VR-based rehabilitation training is a beneficial nonpharmacologic approach for managing mild cognitive impairment or dementia. Immersive VR-based training had greater effects on cognition and motor function than non-immersive VR-based training, but non-immersive VR-based training was more convenient for patients with limitations imposed by their disease. Also, an intervention lasting 5–8 weeks and for >30 min at a frequency of ≥3 times/week achieved the best results. It indicated that a longer intervention cycle may not achieve the best intervention effect and training duration and schedule should be carefully considered when managing patients.
References
1. Aruanno B, Garzotto F. Memholo: mixed reality experiences for subjects with Alzheimer’s disease[J]. Multimed Tools Appl 2019; 78: 13517–13537.
2. Arvanitakis Z, Shah RC, Bennett DA. Diagnosis and management of dementia: review[J]. Jama-Journal of the American Medical Association 2019; 322: 1589–1599.
3. Tangalos EG, Petersen RC. Mild cognitive impairment in geriatrics[J]. Clin Geriatr Med 2018; 34: 563.
4. Jack CR, Albert MS, Knopman DS, et al. Introduction to the recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease[J]. Alzheimers & Dementia 2011; 7: 257–262.
5. Lehrner J, Moser D, Klug S, et al. Subjective memory complaints, depressive symptoms and cognition in patients attending a memory outpatient clinic[J]. Int Psychogeriatr 2014; 26: 463–473.
6. Gaubert F, Borg C, Chainay H. Decision-making in Alzheimer’s disease: the role of working memory and executive functions in the Iowa gambling task and in tasks inspired by everyday situations[J]. Journal of Alzheimers Disease 2022; 90: 1793–1815.
7. Jarry C, Osiurak F, Baumard J, et al. Daily life activities in patients with Alzheimer’s disease or semantic dementia: Multitasking assessment[J]. Neuropsychologia 2021; 150: 107714.
8. Tieri G, Morone G, Paolucci S, et al. Virtual reality in cognitive and motor rehabilitation: facts, fiction and fallacies[J]. Expert Rev Med Devices 2018; 15: 107–117.
9. Son C, Park J. Ecological effects of VR-based cognitive training on ADL and IADL in MCI and AD patients: a systematic review and meta-analysis[J]. Int J Environ Res Public Health 2022; 19: 1660–4601.
10. Garcia-Betances RI, Jimenez-Mixco V, Arredondo MT, et al. Using virtual reality for cognitive training of the elderly[J]. American Journal of Alzheimers Disease and Other Dementias 2015; 30: 49–54.
11. Tuena C, Mancuso V, Stramba-Badiale C, et al. Egocentric and allocentric spatial memory in mild cognitive impairment with real-world and virtual navigation tasks: a systematic review[J]. Journal of Alzheimers Disease 2021; 79: 95–116.
12. Zhu S, Sui Y, Shen Y, et al. Effects of virtual reality intervention on cognition and motor function in older adults with mild cognitive impairment or dementia: a systematic review and meta-analysis[J]. Front Aging Neurosci 2021; 13: 586999.
13. Perra A, Riccardo CL, De Lorenzo V, et al. Fully immersive virtual reality-based cognitive remediation for adults with psychosocial disabilities: A systematic scoping review of methods intervention gaps and meta-analysis of published effectiveness studies[J]. Int J Environ Res Public Health 2023; 20: 1527.
14. Smith CM, Read JE, Bennie C, et al. Can non-immersive virtual reality improve physical outcomes of rehabilitation?[J]. Phys Ther Rev 2013; 17: 1–15.
15. Panerai S, Catania V, Rundo F, et al. Functional living skills in patients with major neurocognitive disorder due to degenerative or non-degenerative conditions: Effectiveness of a non-immersive virtual reality training[J]. Sensors 2023; 23: 1896.
16. Li R, Zhang Y, Jiang Y, et al. Rehabilitation training based on virtual reality for patients with Parkinson’s disease in improving balance, quality of life, activities of daily living, and depressive symptoms: a systematic review and meta-regression analysis[J]. Clin Rehabil 2021; 35: 1089–1102.
17. Ren Y, Lin C, Zhou Q, et al. Effectiveness of virtual reality games in improving physical function, balance and reducing falls in balance-impaired older adults: a systematic review and meta-analysis[J]. Arch Gerontol Geriatr 2023; 108: 104924.
18. Park JH. Does the virtual shopping training improve executive function and instrumental activities of daily living of patients with mild cognitive impairment?[J]. Asian J Psychiatry 2022; 69: 102977.
19. Moher DLAT. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement[J]. PLoS Med 2009; 339: 333–339.
20. Park JH. Does the virtual shopping training improve executive function and instrumental activities of daily living of patients with mild cognitive impairment?[J]. Asian J Psychiatr 2022; 69: 102977.
21. Choi W, Lee S. The effects of virtual Kayak paddling exercise on postural balance, muscle performance, and cognitive function in older adults with mild cognitive impairment: a randomized controlled trial[J]. J Aging Phys Act 2019; 27: 861–870.
22. Hagovska M, Olekszyova Z. Impact of the combination of cognitive and balance training on gait, fear and risk of falling and quality of life in seniors with mild cognitive impairment[J]. Geriatr Gerontol Int 2016; 16: 1043–1050.
23. Hsieh CC, Lin PS, Hsu WC, et al. The effectiveness of a virtual reality-based tai chi exercise on cognitive and physical function in older adults with cognitive impairment[J]. Dement Geriatr Cogn Disord 2018; 46: 358–370.
24. Hughes TFTF, Flatt JDJD, Fu BB, et al. Interactive video gaming compared to health education in older adults with MCI: a feasibility study[J]. Int J Geriatr Psychiatry 2014; 29: 890–898.
25. Kang JM, Kim N, Lee SY, et al. Effect of cognitive training in fully immersive virtual reality on visuospatial function and frontal-occipital functional connectivity in predementia: randomized controlled trial[J]. J Med Internet Res 2021; 23: e24526.
26. Liao YY, Chen IH, Lin YJ, et al. Effects of virtual reality-based physical and cognitive training on executive function and dual-task gait performance in older adults with mild cognitive impairment: a randomized control trial[J]. Front Aging Neurosci 2019; 11: 162.
27. Liao YY, Tseng HY, Lin YJ, et al. Using virtual reality-based training to improve cognitive function, instrumental activities of daily living and neural efficiency in older adults with mild cognitive impairment[J]. Eur J Phys Rehabil Med 2020; 56: 47–57.
28. Man DW, Chung JC, Lee GY. Evaluation of a virtual reality-based memory training programme for Hong Kong Chinese older adults with questionable dementia: a pilot study[J]. Int J Geriatr Psychiatry 2012; 27: 513–520.
29. Mirelman A, Rochester L, Maidan I, et al. Addition of a non-immersive virtual reality component to treadmill training to reduce fall risk in older adults (V-TIME): a randomised controlled trial[J]. Lancet 2016; 388: 1170–1182.
30. Oliveira J, Gamito P, Souto T, et al. Virtual reality-based cognitive stimulation on people with mild to moderate dementia due to Alzheimer’s disease: a pilot randomized controlled trial[J]. Int J Environ Res Public Health 2021; 18: 1–13.
31. Padala KP, Padala PR, Malloy TR, et al. Wii-fit for improving gait and balance in an assisted living facility: a pilot study.[J]. J Aging Res 2012; 2012: 597573.
32. Padala KP, Padala PR, Lensing SY, et al. Home-Based exercise program improves balance and fear of falling in community-dwelling older adults with mild Alzheimer’s disease: a pilot study[J]. J Alzheimers Dis 2017; 59: 565–574.
33. Park JH, Park JH. Does cognition-specific computer training have better clinical outcomes than non-specific computer training? A single-blind, randomized controlled trial[J]. Clin Rehabil 2018; 32: 213–222.
34. Park J, Jung Y, Lee G. Virtual reality-based cognitive–motor rehabilitation in older adults with mild cognitive impairment: a randomized controlled study on motivation and cognitive function[J]. Healthcare (Basel) 2020; 8: 335.
35. Park JH, Liao Y, Kim DR, et al. Feasibility and tolerability of a culture-based virtual reality (VR) training program in patients with mild cognitive impairment: A randomized controlled pilot study[J]. Int J Environ Res Public Health 2020; 17: 3030.
36. Schwenk M, Sabbagh M, Lin I, et al. Sensor-based balance training with motion feedback in people with mild cognitive impairment[J]. J Rehabil Res Dev 2016; 53: 945–958.
37. Thapa N, Park HJ, Yang JG, et al. The effect of a virtual reality-based intervention program on cognition in older adults with mild cognitive impairment: a randomized control trial[J]. J Clin Med 2020; 9: 1–11.
38. Uğur F, Sertel M. The effect of virtual reality applications on balance and gait speed in individuals with Alzheimer dementia: a pilot study[J]. Top Geriatr Rehabil 2020; 36: 221–229.
39. Yang J, Thapa N, Park H, et al. Virtual reality and exercise training enhance brain, cognitive, and physical health in older adults with mild cognitive impairment[J]. Int J Environ Res Public Health 2022; 19: 13300.
40. Zhao Rongrong LGGC. Application of virtual reality technology in cognitive rehabilitation training in patients with mild cognitive impairment. [J]. Neural Injury Funct Reconstruct 2021; 10: 590–592.
41. Tombaugh TN. Trail making test A and B: normative data stratified by age and education[J]. Arch Clin Neuropsychol 2004; 19: 203–214.
42. Kim O, Pang Y, Kim J. The effectiveness of virtual reality for people with mild cognitive impairment or dementia: a meta-analysis[J]. BMC Psychiatry 2019; 19: 219.
43. Maggio MG, Latella D, Maresca G, et al. Virtual reality and cognitive rehabilitation in people with stroke: an overview[J]. J Neurosci Nursing 2019; 51: 101–105.
44. Bourrelier J, Fautrelle L, Haratyk E, et al. Enhancement of anticipatory postural adjustments by virtual reality in older adults with cognitive and motor deficits: a randomised trial.[J]. Geriatrics (Basel, Switzerland) 2021; 6: 1–16.
45. Barrett LF. The theory of constructed emotion: an active inference account of interoception and categorization[J]. Soc Cogn Affect Neurosci 2017; 12: w154.
46. Slater M. Measuring presence: a response to the Witmer and Singer presence questionnaire[J]. Presence-Teleoperators Virtual Environ 1999; 8: 560–565.
47. Subramanian SK, Lourenco CB, Chilingaryan G, et al. Arm motor recovery using a virtual reality intervention in chronic stroke: randomized control trial[J]. Neurorehabil Neural Repair 2013; 27: 13–23.
48. De Luca R, Russo M, Naro A, et al. Effects of virtual reality-based training with BTs-nirvana on functional recovery in stroke patients: preliminary considerations[J]. Int J Neurosci 2018; 128: 791–796.
49. Hao J, He Z, Yu X, et al. Comparison of immersive and non-immersive virtual reality for upper extremity functional recovery in patients with stroke: a systematic review and network meta-analysis[J]. Neurol Sci 2023; 27: 2101417.
50. Clemente M, Rey B, Rodriguez-Pujadas A, et al. A functional magnetic resonance imaging assessment of small animals’ phobia using virtual reality as a stimulus[J]. JMIR Serious Games 2014; 2: e6.
51. Sokolov AA, Collignon A, Bieler-Aeschlimann M. Serious video games and virtual reality for prevention and neurorehabilitation of cognitive decline because of aging and neurodegeneration[J]. Curr Opin Neurol 2020; 33: 239–248.
52. Ge S, Zhu Z, Wu B, et al. Technology-based cognitive training and rehabilitation interventions for individuals with mild cognitive impairment: a systematic review[J]. BMC Geriatr 2018; 18: 213.
53. Liu J, Zhong D, Chen L, et al. Effects of virtual reality cognitive training in individuals with mild cognitive impairment: a systematic review and meta-analysis[J]. Int J Geriatr Psychiatry 2021; 36: 1829–1847.
[BLOG POST] Robot-assisted neurocognitive rehabilitation of the hand
Posted by Kostas Pantremenos in Cognitive Rehabilitation, Paretic Hand, Rehabilitation robotics on February 12, 2023
Authors: Irina Benedek, Oana Vanta
1 Innovations in neurorehabilitation of the upper limbs
2 A multimodal approach to robot-assisted neurocognitive rehabilitation of the hand
4 The roles of robotic devices in improving upper extremity function
5 Results, discussions, and future perspectives
6 Conclusion on robot-assisted neurocognitive rehabilitation of the hand
Innovations in neurorehabilitation of the upper limbs
Is robot-assisted neurocognitive rehabilitation of the hand effective? Stroke is the leading cause of mortality and disability worldwide, negatively impacting the overall quality of life of patients. Most stroke survivors suffer from different types of motor impairments, despite the progressions in the field. Since neurorehabilitation of the upper limbs remains challenging, robotic-assisted therapies have been studied and developed in recent years. These methods were established as safe and practical therapies were used additionally to neurorehabilitation programs [1]. More precisely, in the last two decades, robotic devices that focused on training the proximal upper extremity were assessed, showing results similar to dose-matched conventional therapies [2].
For more information on upper limb neurorehabilitation, visit:
- The feasibility of repetitive sensory stimulation in the rehabilitation of the upper limb after a stroke (the PULSE-I study)
- Wearable elbow robot in rehabilitation after a stroke
- Recovery of precise hand movements after stroke
A multimodal approach to robot-assisted neurocognitive rehabilitation of the hand
Distal arm sensorimotor function is essential for improving the quality of life after a stroke. Nevertheless, the distal arm function is crucial for the ability to perform daily activities and is usually severely affected after a cerebrovascular accident when there is a low probability of regaining its full function [3]. However, scientific data demonstrates the possibility of recovery with intensive motor training provided by robotic-assisted devices [4, 5]. Until now, the focus has been on movement practice without implementing a therapeutic paradigm adapted to the capabilities of the technology in question.
In the study by Ranzani et al. [6] from 2020, the goal was to investigate sensorimotor robotic-assisted rehabilitation of hand function and cognition training in patients with subacute stroke. The researchers based their comprehensive approach on the fact that cognition is essential for appropriate interactions between body and environment, such as [6]:
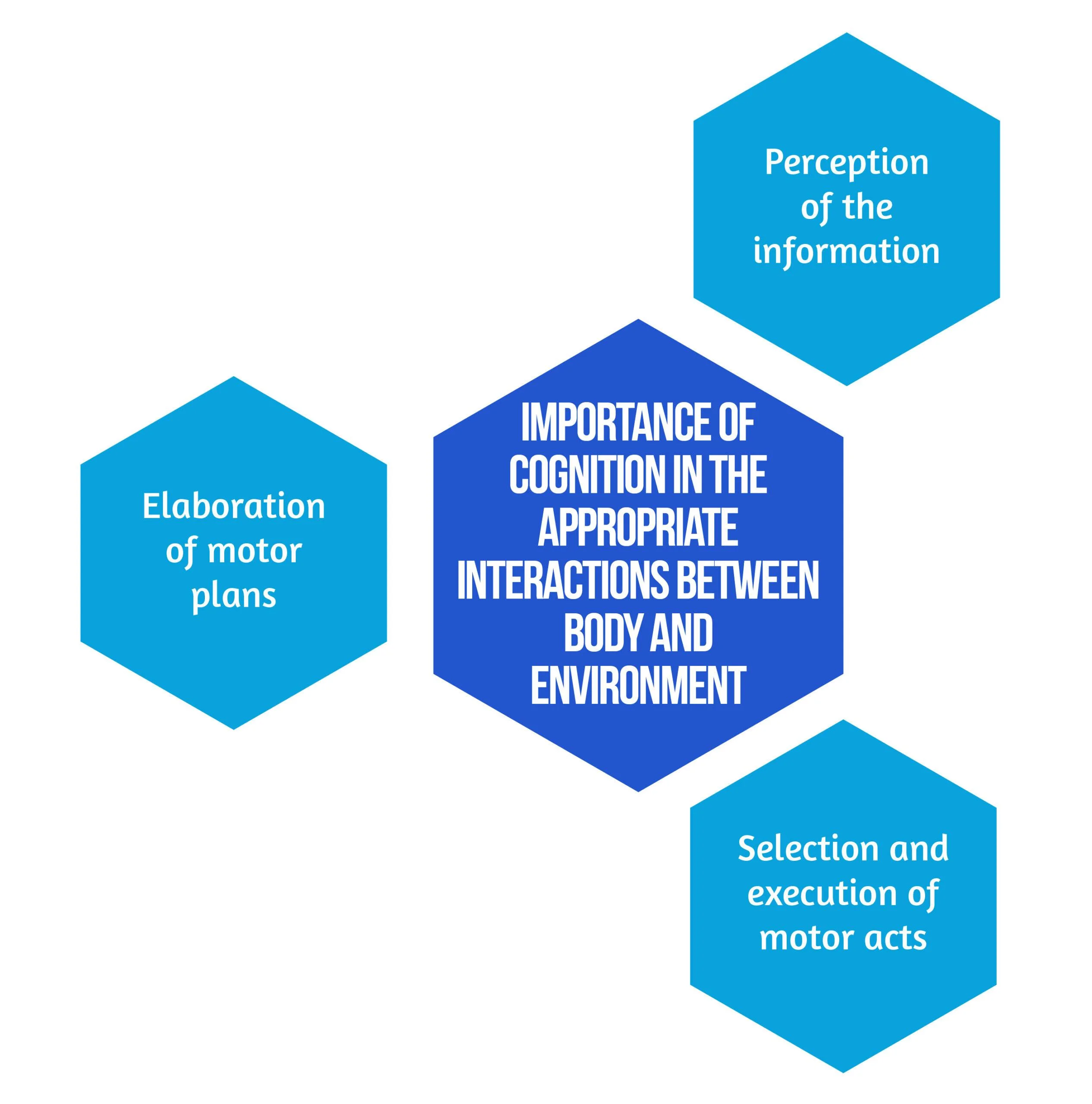
Figure 1. Importance of Cognition
Secondary objectives derived from the hypothesis are that neurocognitive robotic-assisted hand recovery would also improve motor, sensory, and cognitive functions in this category of patients. This method is particularly relevant for hand rehabilitation due to the importance of the cognitive processing of sensory information. Furthermore, combining multimodal inputs requires the involvement of associative cortices that are essential for learning and, consequently, neuronal plasticity and recovery [7]. Even though few studies have compared neurocognitive therapies to other rehabilitation treatments [8, 9], preliminary evidence suggested promising results in the following:
- Enhancing upper limb function
- Improving the ability to conduct daily tasks
- The overall quality of life [8,9].
Inspired by the neurocognitive approach, the authors [6] implemented this concept on a robotic-assisted device focused on exercises including grasping and pronation-supination. Virtual objects were produced by the robot, both visually and haptically, simulating the tangible materials used in traditional recovery [6].
Study design
The study conducted by Ranzani et al. [6] was a randomized control trial that took place in Switzerland. Patients were randomly assigned into two groups: the robot-assisted group (RG), which benefited from neurocognitive therapy with “ReHapticKnob” (Fig. 2), and the control group (CG), which received dose-matched classical neurocognitive therapy. With the main focus on hand function, all participants had three neurocognitive therapy sessions per day over four weeks. Each session lasted 45 minutes, and in the RG, one session was replaced by robot-assisted therapy. The subjects were under the careful supervision of a specialized therapist who adapted the intensity and difficulty level of the exercises according to every patient. The type and number of exercises performed in the RG group were also assessed to match the therapy type and dose performed in conventional recovery programs [6].

Figure 2. Picture of a subject with stroke, using the rehabilitation robot ( available from [6])Between April 2013 and March 2017, 33 patients who suffered a subacute stroke were evaluated and included in the study. Seventeen subjects were randomized in the RG, while 16 were allocated to the CG. 27 patients benefited from the allocated therapy and completed the T1 assessment, while 23 completed the entire protocol. Six patients withdrew before the T1 evaluation due to medical reasons or lack of motivation. No adverse events related to the interventions were noted [6]. The eligibility criteria are described in Figure 3 below:
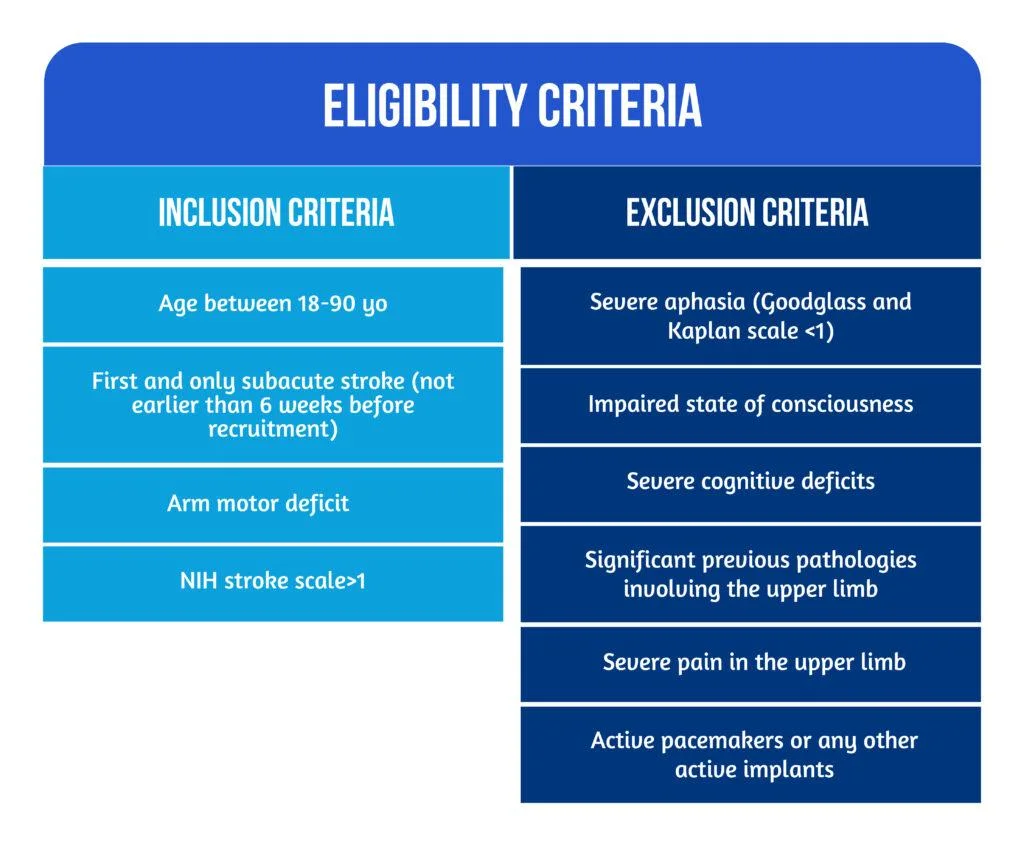
Figure 3. Patient Eligibility
The roles of robotic devices in improving upper extremity function
As mentioned above, the neurocognitive method proposed in this study implied both sensorimotor and cognitive components, which are equally important during the performance of complicated tasks and daily activities. Patients were instructed to explore different objects, such as sponges, sticks, and strings, to discern their properties by using haptic and postural senses. A robotic device is an ideal therapeutic instrument for performing such tasks because it can render a complex range of stimuli in a repeatable, well-controlled manner [10].
The neurorehabilitation program provided in the RG group consisted of seven types of exercises [6], as showcased in Figure 4 below:
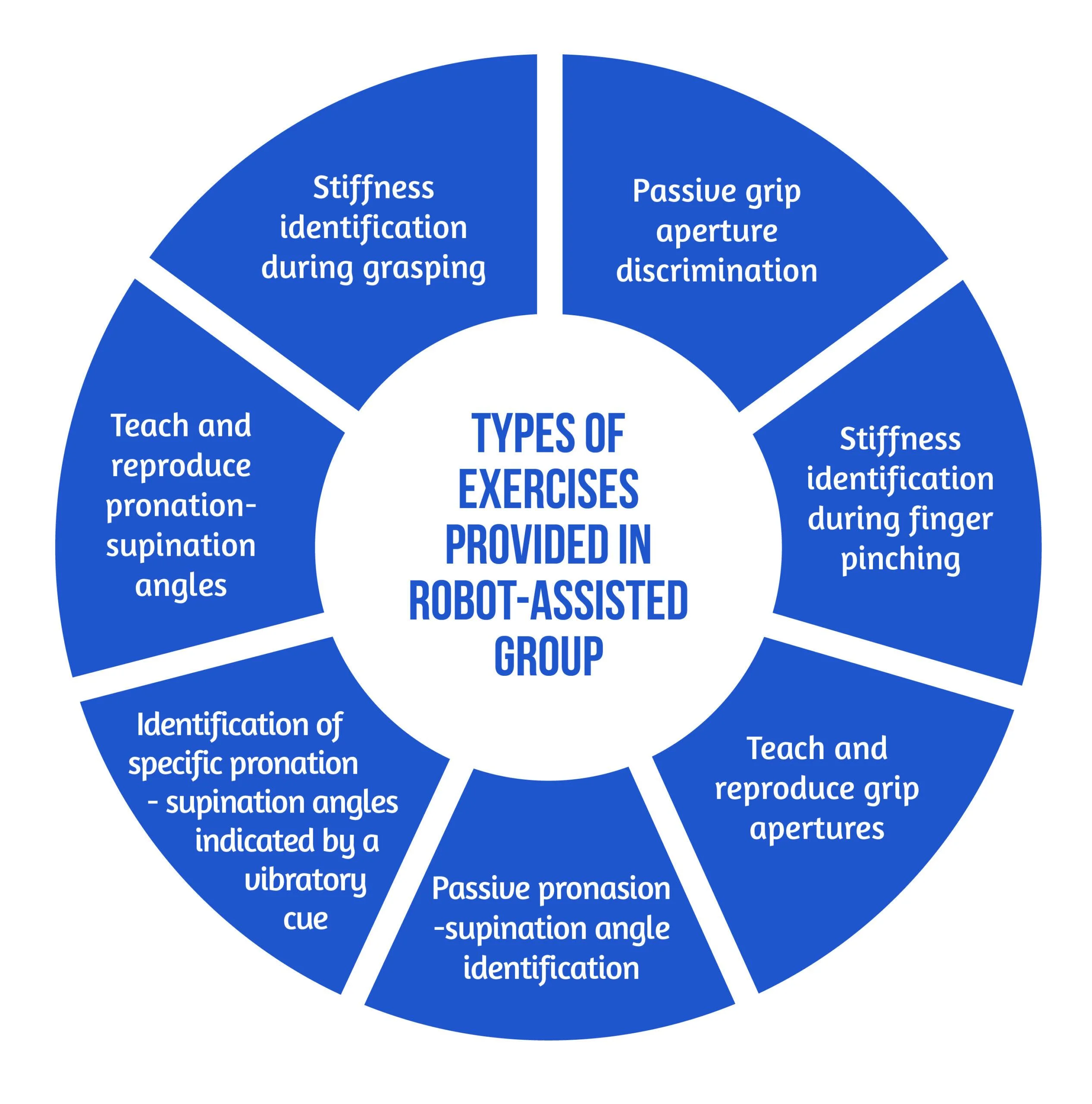
Figure 4. Types of Exercises
The motor aspects of the intervention included symmetric thumb and finger flexion/extension and forearm pronation/supination, executed separately or combined. The sensory aspects included encoding the following types of somatosensory signals without visual information: sponge/spring stiffness, object shape and size, arm positioning, and vibratory cues. The cognitive element of the training, executed passively (with guidance from the robot or therapist) or actively (by the subject), required the elaboration/recognition of perceptual information (object length, stiffness) and encoding/decoding of this information in working memory for comparison purposes of more than one item, along with planning and executing the specific motor plans [6].
The study’s primary outcome was evaluating the changes in upper limb extremity motor impairment from baseline to the end of treatment (T0-T1). The parameter used to quantify the results was the Fugl-Meyer Assessment of the Upper Limb Extremity scale (FMA-UE). Different motor, sensory and cognitive scales were also used for the secondary outcomes. The average number of task repetitions and therapy intensity were assessed to compare the two study groups in terms of dose matching. For the acceptance of the neurocognitive robotic-assisted program, a 4-item questionnaire was developed. The two study groups were evaluated and compared for all outcome measures after the intervention (T-T0) and at the regular follow-ups (T2-T0 and T3-T0) [6].
Results, discussions, and future perspectives
According to the equivalence analysis, the change in FMA-UE in the robotic-assisted group may be regarded as non-inferior to the control group. Both groups showed improvements in all secondary clinical scores after 4 weeks of therapy (T1). In addition to motor impairments, sensory and cognitive deficits were also improved in both groups after 4 weeks of treatment (T1) [6]. However, by the end of the study, subjects randomized in the RG group tended to show better results. At T2 and T3, the changes in FMA-UE were also sustained. When comparing changes in clinical measures over time, the two groups did not demonstrate any significant between-group differences [6].
According to the supervising therapist, the RG did an average of 71.49 task repetitions during a treatment session, compared to 73.47 in the Control Group. There was no statistically significant difference in therapy intensity between the two groups when comparing robot-assisted and traditional therapy sessions [6].
Regarding neurocognitive therapy sessions, the average daily quantity of occupational therapy and/or lower limb kinesiotherapy did not differ statistically between the groups.
The acceptance of the neurocognitive robot-assisted therapy was evaluated by using a self-reported questionnaire answered by 12 patients [6], as showcased in Figure 5:
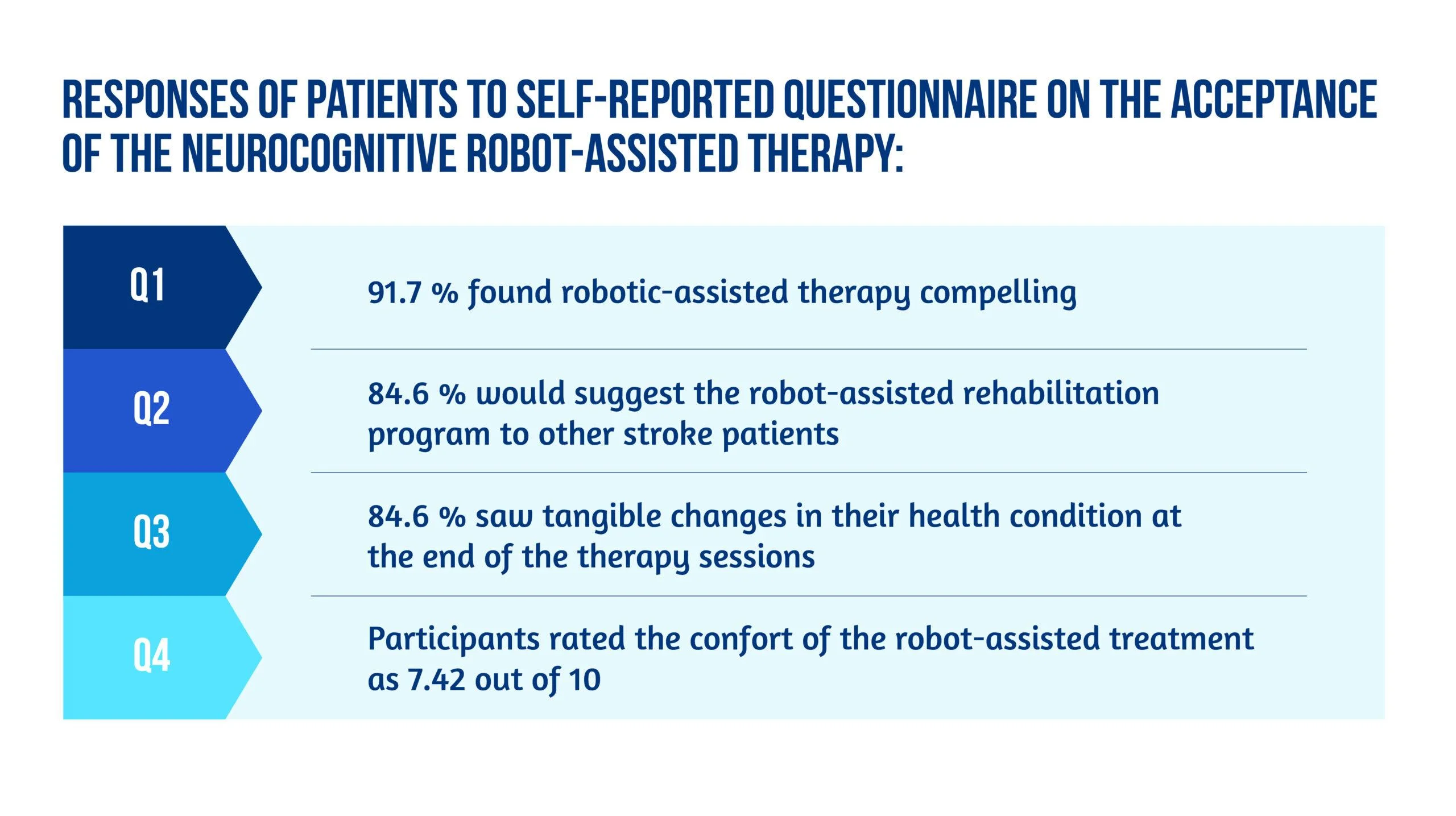
Figure 5. Patient responses
Unlike other robot-assisted rehabilitation studies that focus primarily on locomotor training, this method fully utilized the robot’s haptic rendering capabilities and suggested a therapy regimen tailored to this potential [6].
Ranzani and the team were able to demonstrate that this strategy was well-liked and recommended by the majority of patients. It could also be included in the patient’s daily routines in the subacute stage of stroke recovery. Although the questionnaire pointed out slight pain in finger fixation and difficulty levels were sometimes evaluated as excessively high in three out of seven exercises [6], most subjects considered the program encouraging and pleasant, and they noticed tangible benefits to their health after completing it.
The findings of the equivalency test assessing the evolution in the FMA-UE show that motor recovery in the RG was not inferior to the CG for the specific intervention. Usually, little to no difference between traditional and robotic-assisted therapies should be expected since the dosage and therapeutic movements are meant to be identical between the study groups. Furthermore, the idea that a traditional treatment session might be replaced without compromising the overall rehab programs opens up new possibilities for robot-assisted therapy development [6].
Conclusion on robot-assisted neurocognitive rehabilitation of the hand
This research reported the findings of a randomized controlled trial that aimed to compare robotic-assisted recovery to conventional therapy for patients with a subacute stroke and impairment of hand function. Although the study included a limited number of participants, the results showed that robot-assisted treatments might be successfully incorporated into clinical neurorehabilitation routines [6]. In other words, this trial is also an indicator of how low data can become significant data. Compared to conventional dose-matched neurocognitive programs, the results demonstrated that this innovative approach was at least as practical as the conventional one.
Early exposure of stroke patients to using such patient-tailored robot-assisted therapy programs opens the door to using such technology in the clinic with little therapist supervision or at home after hospital release to help enhance the dose of hand therapy for stroke patients [6].
Bibliography
- Veerbeek JM, Langbroek-Amersfoort AC, van Wegen EE, Meskers CG, Kwakkel G. Effects of robot-assisted therapy for the upper limb after stroke: a systematic review and meta-analysis. Neurorehabil Neural Repair. 2017; 31(2):107–21. doi: 10.1177/1545968316666957
- Maciejasz P, Eschweiler J, Gerlach-Hahn K, Jansen-Troy A, Leonhardt S. A survey on robotic devices for upper limb rehabilitation. J Neuroeng Rehabil. 2014;11(1):3. DOI: 10.1186/1743-0003-11-3
- Fischer HC, Stubblefield K, Kline T, Luo X, Kenyon RV, Kamper DG. Hand rehabilitation following stroke: a pilot study of assisted finger extension training in a virtual environment. Top Stroke Rehabil. 2007;14(1):1–12. DOI: 10.1310/tsr1401-1
- Lambercy O, Dovat L, Yun H, Wee SK et al. Effects of a robot-assisted training of grasp and pronation/supination in chronic stroke: a pilot study. J Neuroeng Rehabil. 2011;8(1):63. doi: 10.1186/1743-0003-8-63.
- Hsieh YW, Lin KC, Wu CY, Shih TY, Li MW, Chen CL. Comparison of proximal versus distal upper-limb robotic rehabilitation on motor performance after stroke: a cluster controlled trial. Sci Rep. 2018;8(1):2091. doi: 10.1038/s41598-018-20330-3
- Ranzani R, Lambercy O, Metzger JC, Califfi A et al. Neurocognitive robot-assisted rehabilitation of hand function: a randomized control trial on motor recovery in subacute stroke. J Neuroeng Rehabil. 2020 Aug 24;17(1):115. doi: 10.1186/s12984-020-00746-7.
- Van de Winckel A, Wenderoth N, De Weerdt W, Sunaert S et al. Frontoparietal involvement in passively guided shape and length discrimination: a comparison between subcortical stroke patients and healthy controls. Exp Brain Res Springer. 2012;220(2):179–89. doi: 10.1007/s00221-012-3128-2
- Sallés L, Martín-Casas P, Gironès X, Durà MJ et al. A neurocognitive approach for recovering upper extremity movement following subacute stroke: a randomized controlled pilot study. J Phys Ther Sci. 2017;29(4):665–72. doi: 10.1589/jpts.29.665
- Lee S, Bae S, Jeon D, Kim KY. The effects of cognitive exercise therapy on chronic stroke patients’ upper limb functions, activities of daily living and quality of life. J Phys Ther Sci. 2015;27(9):2787–91. doi: 10.1589/jpts.27.2787
- Metzger J-C, Lambercy O, Califfi A, Dinacci D et al. Assessment-driven selection and adaptation of exercise difficulty in robotassisted therapy: a pilot study with a hand rehabilitation robot. J Neuroeng Rehabil. 2014;11(1):154. Available at: https://jneuroengrehab.biomedcentral.com/articles/10.1186/1743-0003-11-154
[Abstract] Noninvasive brain stimulation for cognitive rehabilitation following traumatic brain injury: a systematic review
Posted by Kostas Pantremenos in Cognitive Rehabilitation, TBI, tDCS/rTMS on December 6, 2022
Abstract
Traumatic brain injury (TBI) can cause numerous cognitive deficits. These deficits are associated with disability and reduction in quality of life. Noninvasive brain stimulation (NIBS) provides excitatory or inhibitory stimuli to the cerebral cortex. This review aimed to examine the effectiveness of NIBS (i.e., rTMS and tDCS) on cognitive functions in patients with TBI. PubMed, SCOPUS, PEDro, CINAHL, MEDLINE, REHABDATA, and Web of Science were searched from inception to May 2021. The risk of bias in the randomized controlled trials was assessed using the Cochrane Collaboration’s instrument. The Physiotherapy Evidence Database (PEDro) scale was applied to evaluate the risk of bias in the non-randomized controlled trials. Ten studies met our inclusion criteria. Six studies used repetitive Transcranial Magnetic Stimulation (rTMS), and four used transcranial Direct Current Stimulation (tDCS) as cognitive rehabilitation interventions. The results showed heterogenous evidence for the effects of rTMS and tDCS on cognitive function outcomes in individuals with TBI. The evidence for the effects of NIBS on cognition following TBI was limited. TDCS and rTMS are safe and well-tolerated interventions post-TBI. The optimal stimulation sites and stimulation parameters remain unknown. Combining NIBS with traditional rehabilitation interventions may contribute to greater enhancements in cognitive functions post-TBI.
Similar articles
- The Effect of Non-Invasive Brain Stimulation (NIBS) on Executive Functioning, Attention and Memory in Rehabilitation Patients with Traumatic Brain Injury: A Systematic Review.Hara T, Shanmugalingam A, McIntyre A, Burhan AM.Diagnostics (Basel). 2021 Mar 31;11(4):627. doi: 10.3390/diagnostics11040627.PMID: 33807188 Free PMC article. Review.
- The Effect of Non-Invasive Brain Stimulation (NIBS) on Attention and Memory Function in Stroke Rehabilitation Patients: A Systematic Review and Meta-Analysis.Hara T, Shanmugalingam A, McIntyre A, Burhan AM.Diagnostics (Basel). 2021 Feb 3;11(2):227. doi: 10.3390/diagnostics11020227.PMID: 33546266 Free PMC article. Review.
- Noninvasive Brain Stimulation for Rehabilitation of Pediatric Motor Disorders Following Brain Injury: Systematic Review of Randomized Controlled Trials.Elbanna ST, Elshennawy S, Ayad MN.Arch Phys Med Rehabil. 2019 Oct;100(10):1945-1963. doi: 10.1016/j.apmr.2019.04.009. Epub 2019 May 10.PMID: 31078616
- Effects of Noninvasive Brain Stimulation (NIBS) on Cognitive Impairment in Mild Cognitive Impairment and Alzheimer Disease: A Meta-analysis.Wang T, Guo Z, Du Y, Xiong M, Yang Z, Ren L, He L, Jiang Y, McClure MA, Mu Q.Alzheimer Dis Assoc Disord. 2021 Jul-Sep 01;35(3):278-288. doi: 10.1097/WAD.0000000000000464.PMID: 34432674
- Non-invasive brain stimulation techniques for chronic pain.O’Connell NE, Marston L, Spencer S, DeSouza LH, Wand BM.Cochrane Database Syst Rev. 2018 Mar 16;3(3):CD008208. doi: 10.1002/14651858.CD008208.pub4.PMID: 29547226 Free PMC article. Updated. Review.
[BLOG POST] The VR Device For ALL Your Rehab Needs.
Posted by Kostas Pantremenos in REHABILITATION, Virtual reality rehabilitation on November 26, 2022


-Shoulder & Elbow Function
-Grip & Release Strengthening
-Gross Muscle Strengthening
-Upper Extremity Robotic Compatible
-Functional Reaching

-Hip, Knee, & Ankle Function
-Gross Muscle Strengthening
-Bike, Nu-Step, & Standing Frame Compatible
-Weight Bearing & Weight Shifting

-Cervical ROM
-Cervical & Trunk Proprioception
-Vestibular Rehab
-Posture
-Functional Balance & Stability
-Weight Shifting & Weight Bearing

-Chronic/Acute Pain
-Distraction
-Controlled Breathing
-Decompression
-Anxiety Alleviation

-Audio Que Recognition
-Visual-Spatial Awareness
-Attention
-Money Management
-Listening Skills

-Functional Reaching
-Functional Balance and Stability
-Grocery Shopping
-Money Management
[Abstract] Rehabilitation with accurate adaptability walking tasks or steady state walking: A randomized clinical trial in adults post-stroke
Posted by Kostas Pantremenos in Gait Rehabilitation - Foot Drop on August 16, 2022
Abstract
Objective:
To assess changes in walking function and walking-related prefrontal cortical activity following two post-stroke rehabilitation interventions: an accurate adaptability (ACC) walking intervention and a steady state (SS) walking intervention.
Design:
Randomized, single blind, parallel group clinical trial.
Setting:
Hospital research setting.
Subjects:
Adults with chronic post-stroke hemiparesis and walking deficits.
Interventions:
ACC emphasized stepping accuracy and walking adaptability, while SS emphasized steady state, symmetrical stepping. Both included 36 sessions led by a licensed physical therapist. ACC walking tasks recruit cortical regions that increase corticospinal tract activation, while SS walking activates the corticospinal tract less intensely.
Main measures:
The primary functional outcome measure was preferred steady state walking speed. Prefrontal brain activity during walking was measured with functional near infrared spectroscopy to assess executive control demands. Assessments were conducted at baseline, post-intervention (three months), and follow-up (six months).
Results:
Thirty-eight participants were randomized to the study interventions (mean age 59.6 ± 9.1 years; mean months post-stroke 18.0 ± 10.5). Preferred walking speed increased from baseline to post-intervention by 0.13 ± 0.11 m/s in the ACC group and by 0.14 ± 0.13 m/s in the SS group. The Time × Group interaction was not statistically significant (P = 0.86). Prefrontal fNIRS during walking decreased from baseline to post-intervention, with a marginally larger effect in the ACC group (P = 0.05).
Conclusions:
The ACC and SS interventions produced similar changes in walking function. fNIRS suggested a potential benefit of ACC training for reducing demand on prefrontal (executive) resources during walking.
[ARTICLE] Home-based (virtual) rehabilitation improves motor and cognitive function for stroke patients: a randomized controlled trial of the Elements (EDNA-22) system – Full Text
Posted by Kostas Pantremenos in Cognitive Rehabilitation, Paretic Hand, Tele/Home Rehabilitation, Virtual reality rehabilitation on August 14, 2022
Abstract
Background
Home-based rehabilitation of arm function is a significant gap in service provision for adult stroke. The EDNA-22 tablet is a portable virtual rehabilitation-based system that provides a viable option for home-based rehabilitation using a suite of tailored movement tasks, and performance monitoring via cloud computing data storage. The study reported here aimed to compare use of the EDNA system with an active control (Graded Repetitive Arm Supplementary Program—GRASP training) group using a parallel RCT design.
Methods
Of 19 originally randomized, 17 acute-care patients with upper-extremity dysfunction following unilateral stroke completed training in either the treatment (n = 10) or active control groups (n = 7), each receiving 8-weeks of in-home training involving 30-min sessions scheduled 3–4 times weekly. Performance was assessed across motor, cognitive and functional behaviour in the home. Primary motor measures, collected by a blinded assessor, were the Box and Blocks Task (BBT) and 9-Hole Pegboard Test (9HPT), and for cognition the Montreal Cognitive Assessment (MoCA). Functional behaviour was assessed using the Stroke Impact Scale (SIS) and Neurobehavioural Functioning Inventory (NFI).
Results
One participant from each group withdrew for personal reasons. No adverse events were reported. Results showed a significant and large improvement in performance on the BBT for the more-affected hand in the EDNA training group, only (g = 0.90). There was a mild-to-moderate effect of training on the 9HPT for EDNA (g = 0.55) and control (g = 0.42) groups, again for the more affected hand. In relation to cognition, performance on the MoCA improved for the EDNA group (g = 0.70). Finally, the EDNA group showed moderate (but non-significant) improvement in functional behaviour on the SIS (g = 0.57) and NFI (g = 0.49).
Conclusion
A short course of home-based training using the EDNA-22 system can yield significant gains in motor and cognitive performance, over and above an active control training that also targets upper-limb function. Intriguingly, these changes in performance were corroborated only tentatively in the reports of caregivers. We suggest that future research consider how the implementation of home-based rehabilitation technology can be optimized. We contend that self-administered digitally-enhanced training needs to become part of the health literacy of all stakeholders who are impacted by stroke and other acquired brain injuries.
Trial registration Australian New Zealand Clinical Trials Registry (ANZCTR) Number: ACTRN12619001557123. Registered 12 November 2019, http://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=378298&isReview=true
Background
Stroke survivors regard the rehabilitation of upper-limb function as one of the top priorities for increasing their quality of life [1]. However, during the rehabilitation phase the time spent engaged in functional upper-limb activities is often low [2, 3], and at six months after stroke up to 70% remain unable to regain functional use of their affected upper limb(s) [4, 5]. Barriers to therapy include limited access to services, particularly after the transition from acute to in-home care, and low levels of engagement in the rehabilitation program/task itself. These issues have been further compounded by the COVID-19 pandemic, which has highlighted the lack of interventions capable of simultaneously engaging patients in therapy while affording the social distancing and domiciliary options essential for continuity of health care.
Current research shows that optimal recovery from an acquired brain injury (ABI) can be achieved when tailored rehabilitation is provided at high intensity and over a sustained period [7, 8]. Moreover, training tasks should be scaled in complexity (both motor and cognitive) in a manner that accords with the individual needs and capabilities of the patient, fostering motivation and continued progression. To this end, tailored virtual reality (VR), augmented reality (AR) and associated interactive technology can provide a number of key assets for rehabilitation, most notably a medium to increase training doses during critical phases of recovery, scale task difficulty in a systematic way, engage patients’ interest in novel forms of interaction, enhance learning via use of augmented feedback, and record the progress of patients using system-generated metrics. A design principle for many such systems is the notion of enriched therapeutic environments to promote skill acquisition and transfer [9]. The notion here is to present a task environment that not only affords physical movement but also engages the patient’s cognitive attention—both are critical ingredients in skilled performance. This is supported by a recent systematic review and meta-analysis that showed enhanced motor outcomes when these critical ingredients are met with purpose-designed systems [8].
The Elements system (aka EDNA™) [10] was designed originally as a tabletop device (using tangible interfaces) for clinic-based rehabilitation of ABI, with earlier evaluations showing its efficacy for traumatic brain injury [11, 12] and adult stroke [13]. Targeting upper-limb function in TBI patients, significant gains in motor skill were demonstrated in case study [11] and within-groups evaluations [12]. Gains in upper-limb skill also showed positive transfer to everyday function. The most recent RCT extended the application of EDNA to adult stroke and showed strong treatment effects across motor, cognitive and functional outcomes [13]. As well, the experience of using EDNA has been rated highly on the Virtual User Experience Questionnaire (VUE-Q), adapted from the Presence Scale of Witmer and colleagues [14, 15]. Patients have rated highly all six sub-scales: Familiarity, Enjoyment/Engagement, Controllability/Affordance, Efficacy, Social Engagement, and Immersion/Presence, suggesting the system is able to effectively engage the user in the rehabilitation program and promote a sense of improvement. Among the limitations of this in-clinic application, however, is the requirement for one-to-one administration of an adjunct treatment, placing additional demands on the time and resources of rehabilitation services [16].
The capacity to extend access to training into the home environment is particularly important for stroke patients who routinely fail to achieve the recommended doses or durations of therapy necessary to promote meaningful gains [2, 3]. So-called telerehabilitation systems encompass a variety of modalities from videoconferencing, health literacy training delivered over the web, and VR-based systems. Research to date on the benefits of VR- and AR-based therapy in the home are encouraging, but very few controlled trials exist [17]. Evidence suggests, however, that the benefits of such treatment for motor and cognitive function are at least equivalent to standard physical therapy or home-based exercise. In terms of implementation, some guiding principles include the need to “design for engagement” and accommodating the practical challenges of use in the home [18]. These principles speak to the portability of the device and ease of use, providing a viable option for continuity of care as patients transition from the hospital to the home [19].
The EDNA system has therefore recently been extended to include a transportable, tablet device (EDNA-22) for home-based delivery. Targeting upper-limb impairments, the system is designed to provide a viable and flexible therapy option in multiple environments (e.g., clinic, home, and community), which is critical if patients are to achieve recommended doses and durations of rehabilitation [20]. A customised and regular schedule of therapy is delivered via the internet to the patient in their residence, performance data is collected and stored in the cloud, and adherence and performance data is relayed back to the therapist in the clinic. The broad aim of the study presented here was to evaluate the motor, cognitive and functional outcomes of an intensive course of home-based rehabilitation using the EDNA-22 system. First, based on our earlier clinical trials, we expected that participants recovering from stroke would be able to engage effectively in the home-based therapy and adhere to the complete course (i.e., minimum 3 sessions per week, recorded with written log). Second, we expected that the course of therapy would produce significant gains in motor and cognitive function, measured using standardized and validated clinical tools, with the magnitude of changes greater than that observed for an active control therapy (Graded Repetitive Arm Supplementary Program—GRASP) [21, 22]. Third, we expected that patients would also report positive changes in their level of motor functioning. Fourth, we expected that caregivers would report positive change in the general everyday function of patients as a result of the therapy. Finally, we expected the training benefits of EDNA to be maintained across motor, cognitive and functional outcomes at a short-term follow-up (3 months).[…]

[Abstract] The effects of exercise on cognition post-stroke: are there sex differences? A systematic review and meta-analysis
Posted by Kostas Pantremenos in Cognitive Rehabilitation on April 17, 2022
Abstract
Purpose: The aim of this systematic review was to investigate if sex moderated the effect of exercise on cognition in adults post-stroke.
Methods: A systematic review was conducted of randomized controlled trials that involved adults ≥18 years with stroke, any exercise intervention, and reported any outcome related to cognitive function. We compared effect sizes of cognitive outcomes between studies of lower and higher proportion of females (CRD42018092757).
Results: The effects of exercise did not differ between studies of higher and lower female proportions with respect to memory (χ2 =1.52, p = 0.22), executive function (χ2 = 0.56, p = 0.45; Chi2 = 0.00, p = 0.98), language (Chi2 = 3.17, p = 0.08) or global cognition (χ2 = 0.88, p = 0.35).
Conclusion: There were no sex differences in the effects of exercise on memory, executive functioning, language or global cognition in individuals with stroke. Further research is warranted to address sex differences in individuals with stroke to enable better targeting, prevention, and interventions in stroke rehabilitation.IMPLICATIONS FOR REHABILITATIONUnderstanding sex differences and potentially similarities in the relationship between exercise and cognition is an important step in enhancing stroke rehabilitation and the development of optimal, sex-specific rehabilitation.Although our findings suggest that there is no clear rationale for incorporating sex into our clinical decision making, it is still imperative to consider sex factors in research and report results in the literature disaggregated by sex to help inform clinical practice.
Similar articles
- Sex differences in exercise efficacy to improve cognition: A systematic review and meta-analysis of randomized controlled trials in older humans.Barha CK, Davis JC, Falck RS, Nagamatsu LS, Liu-Ambrose T.Front Neuroendocrinol. 2017 Jul;46:71-85. doi: 10.1016/j.yfrne.2017.04.002. Epub 2017 Apr 22.PMID: 28442274 Review.
- Cognitive rehabilitation for executive dysfunction in adults with stroke or other adult non-progressive acquired brain damage.Chung CS, Pollock A, Campbell T, Durward BR, Hagen S.Cochrane Database Syst Rev. 2013 Apr 30;2013(4):CD008391. doi: 10.1002/14651858.CD008391.pub2.PMID: 23633354 Free PMC article. Review.
- Effects of Exercise Training Interventions on Executive Function in Older Adults: A Systematic Review and Meta-Analysis.Chen FT, Etnier JL, Chan KH, Chiu PK, Hung TM, Chang YK.Sports Med. 2020 Aug;50(8):1451-1467. doi: 10.1007/s40279-020-01292-x.PMID: 32447717 Free PMC article.
- Feasibility of a 6-month exercise and recreation program to improve executive functioning and memory in individuals with chronic stroke.Rand D, Eng JJ, Liu-Ambrose T, Tawashy AE.Neurorehabil Neural Repair. 2010 Oct;24(8):722-9. doi: 10.1177/1545968310368684. Epub 2010 May 11.PMID: 20460494 Free PMC article.
- Sex differences in the effects of exercise on cognition post-stroke: Secondary analysis of a randomized controlled trial.Khattab S, Eng JJ, Liu-Ambrose T, Richardson J, MacDermid J, Tang A.J Rehabil Med. 2020 Jan 2;52(1):jrm00002. doi: 10.2340/16501977-2615.PMID: 31608416
[WEB] Could Intermittent Fasting Enhance Your Cognitive Ability?
Posted by Kostas Pantremenos in Cognitive Rehabilitation on April 13, 2022
Submitted by Joshua Schnell

There’s a lot out there on the interweb about the tangible benefits of intermittent fasting, but what’s the science say? Let’s dig in.
In 10 seconds?
Researchers have found that intermittent fasting – a method used by many to lose weight – has made lab mice sharper thinkers – at least in finding an escape route in a maze test. But does it translate to humans?
I heard that fasting makes you more focused?
Well, many people believe so. San Francisco-based biohacking and ‘cognitive modulation’ firm HVMN got into the news a few years ago because the whole team engaged in regular intermittent fasting (IF), saying their most productive day was when they didn’t eat at all and that they felt “clarity and calmness”.
OK, but where’s the scientific proof?
Several studies emerged in the last decade about the physical benefits of intermittent fasting but only a few focused specifically on how it affects cognition, i.e. our thinking process. A recent study tried to fill this gap. Scientists split mice into three groups: one was allowed to feed whenever, another was free to eat but with a 10% calorie restriction, and the third was only allowed food every second day. Then, the animals were put in a water maze designed to assess spatial learning in rodents and had to swim and find an escape platform. After 10 days, the intermittent fasting mice started to outperform the other groups by finding routes to safety faster.
OK, it’s one study, any other proof?
A review highlighted that an intermittent fasting regime – again in lab animals – had several cognitive benefits. No, not ‘smarter thinking’ but improved spatial memory, associative memory, and working memory. Cognitive benefits were also reported in humans, in a clinical trial, involving older adults with mild cognitive issues. A year-long intermittent fasting regimen improved the subjects’ global cognition, verbal memory, and executive function. And, another clinical trial reported a ‘significant improvement’ in the working memory of older people after two years of daily intermittent fasting. The “maze study” also came up with an interesting result: the activity of a cognition-related gene, Klotho, increased in “fasting” mice. This gene is present in humans too, so it’s likely to be the subject of further studies, that could establish whether IF really improves cognition or just slows down decline.
Are there any other beneficial effects of IF on the mind?
We have evidence about the brain and more precisely, neurons – the messengers in our grey matter. According to a review paper, fasting enables cell recovery in neurons. During the fasting period, the recovery “switch” is on, supporting chemical reactions aiding the survival of neurons. During the feeding period, the switch is off helping growth. The same study concluded that fasting improved cognition and slowed down neurodegradation and cognitive decline due to aging, however emphasizing the need for more human studies.
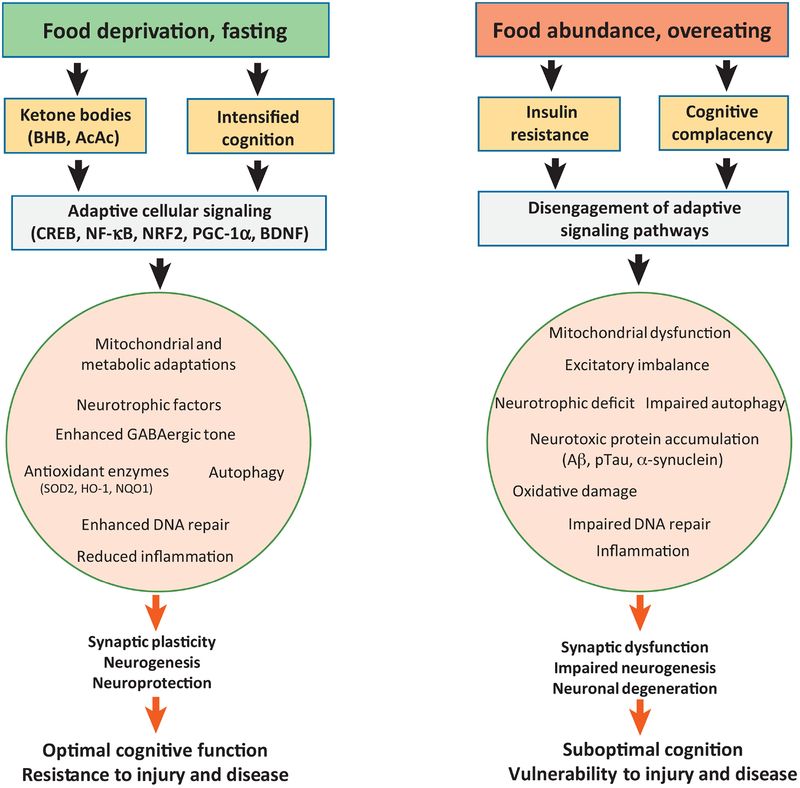
OK, and how is intermittent fasting different from fasting?
IF is not about not eating at all but a combination of ‘feeding windows’ and fasting, either within a day or on certain days. According to this review, it has proven short-to-medium term benefits on glucose and lipid metabolism. The inspiration harks back to prehistoric times when our bodies adapted to the scarcity of food allowing us to function at a high physical and mental level. Other studies suggest that intermittent fasting lowers the risk of diabetes and cardiovascular disease while helping you along the way to achieve and keep a lower body weight – achieving between 0.8 to 13% of weight loss.
Can I really develop a six-pack with this method?
I thought you were interested in whether you become a sharper thinker! OK, let’s say you can step on the right path and not pile on the pounds again. A study from 2015 reported one can drop 3–7% of body weight in a 12-week program. And, researchers comparing a high-protein, low-calorie intermittent fasting plan to a weight loss plan developed for obese individuals found that the intermittent fasting group would regain less body mass, even after a year.
So, should I skip breakfast to ‘eat in a window’?
Only if you’re disciplined! Satisfying your cravings in your eating window with sugary foods will not let you achieve the ‘clarity, calmness, and productivity’ preached by those Californian biohackers. Remember also, that intermittent fasting has not been proven to offer significantly better aesthetic results than other diets.
What is an intermittent fasting plan?
The simplest method is restricting your eating every day to an 8-hour window if you’re a male and a 10–12 hour window if you’re a female.
For lifestyle and social reasons (i.e. dinner with friends or family) many followers simply skip breakfast and only eat from lunchtime.
The ‘human enhancers’ at HVMN signed up for a somewhat tougher regime. The idea is not to eat at all between Monday evening and Wednesday morning.
Their CEO has set up a group and managed to recruit hundreds of followers sharing in his team’s passion for fasting.
[Abstract] The preliminary effects of moderate aerobic training on cognitive function in people with TBI and significant memory impairment: a proof-of-concept randomized controlled trial
Posted by Kostas Pantremenos in Cognitive Rehabilitation, TBI on March 23, 2022
Abstract
This single-blinded RCT investigated cognitive effects of aerobic exercise in persons with TBI-related memory impairment. Five participants . were randomly assigned to 12-weeks of either supervised moderate intensity aerobic cycling or an active control. Outcome measures included neuropsychological assessments and structural neuroimaging (MRI,). The exercise group demonstrated greater improvements on auditory verbal learning (RAVLT; d=1.54) and processing speed (SDMT; d=1.58). The exercise group showed larger increases in volume of the left hippocampus (d=1.49) and right thalamus (d=1.44). These pilot data suggest that 12-weeks of moderate intensity aerobic cycling may improve memory and processing speed in those with TBI-related memory impairments.
Similar articles
- Effects of walking exercise training on learning and memory and hippocampal neuroimaging outcomes in MS: A targeted, pilot randomized controlled trial.Sandroff BM, Wylie GR, Baird JF, Jones CD, Diggs MD, Genova H, Bamman MM, Cutter GR, DeLuca J, Motl RW.Contemp Clin Trials. 2021 Nov;110:106563. doi: 10.1016/j.cct.2021.106563. Epub 2021 Sep 5.PMID: 34496278 Clinical Trial.
- Study protocol: improving cognition in people with progressive multiple sclerosis: a multi-arm, randomized, blinded, sham-controlled trial of cognitive rehabilitation and aerobic exercise (COGEx).Feinstein A, Amato MP, Brichetto G, Chataway J, Chiaravalloti N, Dalgas U, DeLuca J, Feys P, Filippi M, Freeman J, Meza C, Inglese M, Motl RW, Rocca MA, Sandroff BM, Salter A, Cutter G; CogEx Research Team.BMC Neurol. 2020 May 22;20(1):204. doi: 10.1186/s12883-020-01772-7.PMID: 32443981 Free PMC article. Clinical Trial.
- Exercise training effects on memory and hippocampal viscoelasticity in multiple sclerosis: a novel application of magnetic resonance elastography.Sandroff BM, Johnson CL, Motl RW.Neuroradiology. 2017 Jan;59(1):61-67. doi: 10.1007/s00234-016-1767-x. Epub 2016 Nov 26.PMID: 27889837 Clinical Trial.
- Aerobic exercise to improve cognitive function in older people without known cognitive impairment.Young J, Angevaren M, Rusted J, Tabet N.Cochrane Database Syst Rev. 2015 Apr 22;(4):CD005381. doi: 10.1002/14651858.CD005381.pub4.PMID: 25900537 Review.
- Physical activity and enhanced fitness to improve cognitive function in older people without known cognitive impairment.Angevaren M, Aufdemkampe G, Verhaar HJ, Aleman A, Vanhees L.Cochrane Database Syst Rev. 2008 Apr 16;(2):CD005381. doi: 10.1002/14651858.CD005381.pub2.PMID: 18425918 Updated. Review.