Posts Tagged motor rehabilitation
[WEB] Invasive electrical stimulation for stroke treatment
Posted by Kostas Pantremenos in REHABILITATION on March 19, 2024

The CorTec Brain Interchange system is a potential tool to improve motor rehabilitation after stroke (Schuettler, 2023). Here, it is sketched how results from studies of other groups are combined to form CorTec’s vision of a new therapy and how first human data was collected to prove the systems therapy capability
In 1949, the neurophysiologist Donald Hebb postulated “Neurons that fire together, wire together,” which became the theoretical fundament for mechanisms underlying motor rehabilitation, e.g., after stroke. Co‐activation of neurons solidifies and even strengthens their synaptic connections. This “learning” can be utilised in conventional, occupational, or physio‐therapeutic rehabilitation approaches. It has been successfully investigated in animal models for decades that electrical stimulation can facilitate this learning process (Bao, 2020). These studies led to the following practical patient studies on stroke rehabilitation (amongst others):
Practical approaches A: Invasive electrical stimulation for stroke treatment
Deep brain stimulation of the cerebellum was applied in combination with conventional rehabilitation in a Phase I study supported by Enspire DBS Therapy, Cleveland, U.S., on 12 patients to treat impairment of the upper extremity. The median improvement was measured to be 7 points in the Upper‐Extremity Fugl‐Meyer Assessment (UEFMA), a scale ranging from 0 (no function) to 66 (no functional deficit). The patients with partial preservation of the distal motor function improved by 15 points UEFMA. (Baker, 2023)
Cortical stimulation was applied in Phase I (8 patients) and Phase II (24 patients) trials, paired with conventional rehabilitation. Clinically meaningful improvements in arm function (Phase I: +10 points UEFMA vs +1.9 in the control group, Phase II: +5.5 points vs +1.9) were achieved (Plow 2014). However, in Phase III (146 patients) supported by Northstar Neuroscience, Seattle, U.S., no significant differences between stimulated patients and control group were found.
Retrospectively, various aspects of the study design are attributed to the surprising negative outcome, including methods of implantation location identification, and considerations on the geometry of peri‐infarct tissue. (Plow, 2009)
Stimulation of the vagus nerve can modulate brain activity, as the vagus nerve fibers project to the brain. In a pilot study funded by MicroTransponder, Austin, U.S., the stimulation was applied in a pivotal study involving 108 patients in combination with conventional rehabilitation to restore upper extremity function. The UEFMA improved in the stimulated patients by 5 points, while the control group improved by 2.4 points (Dawson, 2021).
Practical approaches B: Non-invasive transcranial magnetic brain stimulation (TMS)
TMS utilises electrical coils to push a transient magnetic field into a target tissue, causing eddy currents to be generated in a localised area.
Effectively, this can be considered electrical tissue stimulation without implanted electrodes. It was demonstrated that TMS applied to the brain can facilitate the excitability of neurons, one aspect that contributes to (re‐)learning. This effect can be modulated when applied in a closed‐ loop manner, e.g. when applied in synchrony to brain activity of selected neural networks.
Depending on the selected brain activity rhythm (wave) and the phase angle between the brain wave and stimulus, neural excitability can be up‐ or down‐regulated (Wischnewski, 2022). If up‐regulated, the excitability can be substantially higher compared to an open‐loop TMS (Gharabaghi, 2014).
Lessons learned/conclusion
Electrical stimulation using an implanted device can substantially enhance stroke recovery. Increasing the excitability of brain tissue is most effective when applied in correct synchronicity with brain activity. Combining these two discoveries will unleash new powers in stroke rehabilitation.
First performance tests on humans using the Brain Interchange system
First of all, the system must be capable of continuously recording human brain activity. We anticipate a daily session of up to two hours in combination with physiotherapy.
In a study carried out with the University of Houston, U.S., we demonstrated 24‐hour recording capability in clinical settings by trying to identify spikes in brain signals of epilepsy patients. The results (number of spikes detected) were compared to those of common clinical amplifiers connected to the patient‐implanted electrodes simultaneously. No significant difference in spike detection was observed across the devices (Ayyoubi, 2024).
Furthermore, it was verified with the University of Washington, U.S., that stimulation artefacts do not compromise the system. Electrical stimulation pulses are accompanied by large electrical signals (artefacts), which can be mistaken by the algorithm for a brain wave, causing erratic triggering of further pulses or masking the brain signals of interest. In two of two patients with implanted electrodes, a closed‐loop phased‐dependent stimulation in synchrony to beta activity was successfully conducted (publication in preparation).
Stroke therapy: Outlook
Scientific background, practical clinical studies and first own performance studies with patients of other groups indicate that the Brain Interchange enables a powerful new stroke therapy.
The first step towards the therapy will be starting an early feasibility study later in 2024 with our clinical partners from the University of Washington in Seattle.
References
- Schuettler, Martin. “CorTec’s Brain InterchangeTM system: Revolutionizing brain therapy with closed-loop neuromodulation”, Open Access Government, July, (2023), pp.164-165.
- Bao, Shi-chun, et al. “Rewiring the lesioned brain: electrical stimulation for post-stroke motor restoration.” Journal of Stroke 22.1 (2020): 47.
- Plow, Ela B., et al. “Invasive cortical stimulation to promote recovery of function after stroke: a critical appraisal.” Stroke 40.5 (2009): 1926-1931.
- Plow, Ela B., and Andre Machado. “Invasive neurostimulation in stroke rehabilitation.” Neurotherapeutics 11 (2014): 572-582.
- Baker, Kenneth B., et al. “Cerebellar deep brain stimulation for chronic post-stroke motor rehabilitation: a phase I trial.” Nature Medicine 29.9 (2023): 2366-2374.
- Dawson, Jesse, et al. “Vagus nerve stimulation paired with rehabilitation for upper limb motor function after ischaemic stroke (VNS-REHAB): a randomised, blinded, pivotal, device trial.” The Lancet 397.10284 (2021): 1545-1553.
- Wischnewski, Miles, et al. “The phase of sensorimotor mu and beta oscillations has the opposite effect on corticospinal excitability.” Brain Stimulation 15.5 (2022): 1093-1100.
- Gharabaghi, Alireza, et al. “Coupling brain-machine interfaces with cortical stimulation for brain-state dependent stimulation: enhancing motor cortex excitability for neurorehabilitation.” Frontiers in Human Neuroscience 8 (2014): 122.
- Ayyoubi, Amir Hossein, et al. “Benchmarking signal quality and spatiotemporal distribution of interictal spikes in prolonged human iEEG recordings using CorTec wireless brain interchange.” Scientific Reports 14.1 (2024): 2652.
[ARTICLE] Design recommendations for XR-based motor rehabilitation exergames at home – Full Text
Posted by Kostas Pantremenos in Video Games/Exergames on January 24, 2024
Introduction: Acquired brain injuries pose significant societal and individual challenges worldwide. The adoption of XR technologies presents an opportunity to enhance current rehabilitation procedures. However, a comprehensive understanding of the specific requirements of different user groups in XR-based rehabilitation remains incomplete. Our objective was to identify design recommendations for designers and researchers of XR-based exergames for motor rehabilitation for lower-limb motor recovery at home.
Methods: After initially conducting a mini-literature review and brief market analysis, we used a human-centered design process, interviewing central stakeholders to understand their perspectives and using thematic analysis to identify recurring themes and insights related to XR-based rehabilitation.
Results: The resulting eight key themes for integrating XR-based exergames into acquired brain injuries (ABI) rehabilitation were safety, flexibility, efficacy, usability, technology, motivation, ownership, and social factors.
Conclusion: By addressing technical and user-oriented demands, our resulting design recommendations aid designers in developing meaningful XR-based rehabilitation exercises.
Introduction
Rapid developments in entertainment technologies have made immersive gaming based on extended reality (XR) increasingly accessible and enjoyable for the general public. However, these technologies also present huge opportunities for other domains, such as medical rehabilitation. In the field of rehabilitation, an increasing number of people suffer from acute brain injuries, which pose individual and societal challenges associated with support and treatment. Consequently, there is a strong demand for novel technological solutions. Integrating the widely available XR-based technologies in rehabilitation processes has the potential to facilitate and promote it. However, the individual requirements on XR-based technologies of all involved user groups still need to be better understood and, therefore, need closer examination. Thus, this article explores those individual requirements for developing user-centric XR-exergames in motor rehabilitation.
Injuries to the brain can result in various long-lasting disabilities due to the organ’s complexity. Those disabilities range from indiscernible symptoms, as the brain can compensate for some damage, to a combination of movement, sensory, emotional, and cognitive disabilities (Castor and El Massioui, 2018). Consequently, individuals affected by brain injuries often face significant difficulties performing daily activities independently and may experience social isolation (Demakis, 2007).
Acquired brain injuries (ABIs), including strokes and traumatic brain injuries (TBIs), are prevalent conditions, with a combined 81 million cases occurring each year (Dewan et al., 2018; Lindsay et al., 2019). Besides the individual tragedy ABIs cause, the economic burden on society for TBIs alone is estimated to be US$ 400 billion (as of 2017) globally (Maas et al., 2017), underlining the importance of more cost-effective rehabilitation procedures in the future.
Although stroke and TBIs differ in pathology and population, they share similarities regarding the resulting neurologic disorders and the subsequent rehabilitation procedure. Mainly the injury’s size, location, and severity are determinants of the experienced disabilities (Castor and El Massioui, 2018).
In summary, ABIs are a common and complex pathology with grave consequences for a single individual and a considerable socio-economic impact. Thus, the therapy process for ABI patients to restore lost functionality and reintegrate them into society is of high priority. However, this process is complicated due to the inherent complexity and pathology of the brain.
Central to the therapy of ABIs is the brain’s inherent capability to adapt and reorganize to compensate for some structural damages and regain lost functions. This process is also known as neuroplasticity. In the best case, neuroplastic processes can lead to a spontaneous recovery after an injury (Hatem et al., 2016). Nevertheless, this process requires external assistance and guidance to rehabilitate from related disabilities sufficiently. In traditional rehabilitation, the direct interaction between therapist and patient is indispensable throughout all rehabilitation phases. Despite its importance, this approach becomes economically impracticable with growing patient numbers and a decreasing healthcare workforce. Consequently, there is an ongoing effort to develop novel technologies to relieve therapists and improve the rehabilitation process. However, most research in motor rehabilitation focuses on improving upper limb functionality, and younger age groups with unique needs, capabilities, and interests are often overlooked (Rudberg et al., 2020; Holloway et al., 2022).
Only a few days after receiving ABI, the patient usually starts with intensive rehabilitation at a hospital or other medical facilities for several weeks. The patient is cared for there by a multidisciplinary team of medical professionals (Turner-Stokes, Sykes, and Silber, 2008). Physiotherapists and occupational therapists play a crucial role in the rehabilitation of motor and sensory impairments. Physiotherapists treat fundamental disabilities of movement, balance, and coordination, whereas occupational therapists assist in relearning higher-level task-specific functions (Govender and Kalra, 2007; Studer, 2007).
After regaining basic abilities, the patient is moved to outpatient units to provide regular supervised therapy while living at home. The training is transferred to the patient’s home, where the patients themself are responsible for following the advised training regime (Cullen et al., 2007; Young and Forster, 2007; Maas et al., 2017). The recovery often stagnates during the later stages of the rehabilitation process (sequela stage). Additionally, the training intensity usually decreases as prolonged, frequent supervised training is not economically viable. Besides the absence of motivational support, the patient receives less corrective feedback in this phase, leading to maladaptive neuroplastic changes and potentially reversing previous improvements (Maas et al., 2017).
Exergames in physical rehabilitation are a type of serious game that aims to facilitate motor rehabilitation through physical play, other than pure entertainment. Over the last few years, they have become a valuable tool for rehabilitation, as the automatization of the training relieves healthcare providers and facilitates home rehabilitation. Those games can guide a training exercise for motor rehabilitation and sometimes give feedback on execution quality (Rüth et al., 2023). To do so, the game input must reliably track the patients’ movements and consider the user’s specific needs and goals. Standard tracking devices are camera systems, balance boards (e.g., Wii fit), and accelerometers (e.g., VR headset and controller) (Gómez-Portes et al., 2021; Rüth et al., 2023).
Ongoing research investigates various technologies that can supplement or improve the current rehabilitation process. Especially promising are extended reality (XR) systems, such as virtual reality (VR), augmented reality (AR), and mixed reality (MR), due to their improved usability, accessibility, and ubiquitousness over the last few years. With the help of a head-mounted device (HMD), the users of such systems can run various applications that allow for the experience of a fully immersive virtual world environment. Sensors integrated into the HMD track the user’s head movement and can be supplemented with additional controllers, body trackers, headphones, or other feedback devices (Mathew and Pillai, 2020).
In this study, we aimed to discover how XR-based exergames can be employed for motor rehabilitation and how this can be sustainably incorporated into the rehabilitation ecosystem. This was done using human-centered design (HCD) approaches and methods to uncover the target user’s needs and requirements associated with motor rehabilitation using co-creation. Besides incorporating current research findings on XR-based exergaming for motor rehabilitation and commercial solutions, we interviewed stakeholders, such as subject matter experts, healthcare professionals, and patients. Based on the various knowledge streams, we developed design recommendations that can assist Human-Computer Interaction designers in understanding the respective stakeholders’ needs and in developing future XR-based lower limb rehabilitation applications with a particular focus on in-home treatment.
The article is organized as follows: First, we summarize the results of a brief review of the current state-of-the-art XR-based rehabilitation technology. Next, we present findings and resulting themes from interviews with subject matter experts, therapists, and ABI patients. These findings are presented as a general patient journey and exemplified through a specific patient scenario. We then discuss our findings and present design recommendations before concluding the article. […]

[ARTICLE] Brain oscillations in reflecting motor status and recovery induced by action observation-driven robotic hand intervention in chronic stroke – Full Text
Posted by Kostas Pantremenos in Paretic Hand, REHABILITATION, Rehabilitation robotics on December 17, 2023
Hand rehabilitation in chronic stroke remains challenging, and finding markers that could reflect motor function would help to understand and evaluate the therapy and recovery. The present study explored whether brain oscillations in different electroencephalogram (EEG) bands could indicate the motor status and recovery induced by action observation-driven brain–computer interface (AO-BCI) robotic therapy in chronic stroke. The neurophysiological data of 16 chronic stroke patients who received 20-session BCI hand training is the basis of the study presented here. Resting-state EEG was recorded during the observation of non-biological movements, while task-stage EEG was recorded during the observation of biological movements in training. The motor performance was evaluated using the Action Research Arm Test (ARAT) and upper extremity Fugl–Meyer Assessment (FMA), and significant improvements (p < 0.05) on both scales were found in patients after the intervention. Averaged EEG band power in the affected hemisphere presented negative correlations with scales pre-training; however, no significant correlations (p > 0.01) were found both in the pre-training and post-training stages. After comparing the variation of oscillations over training, we found patients with good and poor recovery presented different trends in delta, low-beta, and high-beta variations, and only patients with good recovery presented significant changes in EEG band power after training (delta band, p < 0.01). Importantly, motor improvements in ARAT correlate significantly with task EEG power changes (low-beta, c.c = 0.71, p = 0.005; high-beta, c.c = 0.71, p = 0.004) and task/rest EEG power ratio changes (delta, c.c = −0.738, p = 0.003; low-beta, c.c = 0.67, p = 0.009; high-beta, c.c = 0.839, p = 0.000). These results suggest that, in chronic stroke, EEG band power may not be a good indicator of motor status. However, ipsilesional oscillation changes in the delta and beta bands provide potential biomarkers related to the therapeutic-induced improvement of motor function in effective BCI intervention, which may be useful in understanding the brain plasticity changes and contribute to evaluating therapy and recovery in chronic-stage motor rehabilitation.
1 Introduction
Stroke has been the leading cause of acquired disability in adults globally for decades (Mendis, 2013). Although the mortality rate declined with improved healthcare, approximately 80% of stroke victims still experience motor impairment, and more than 30% of patients suffer despite intensive rehabilitation (Lai et al., 2002; Young and Forster, 2007). It is worse for the chronic group with severe motor impairments in the upper limbs. On the one hand, effective interventions like constraint-induced movement therapy (CIMT) may not be applicable to those patients without enough residual active movement (Thrasher et al., 2008). On the other hand, motor recovery in chronic stroke is more challenging due to the decreasing plasticity of spontaneous recovery (Cassidy and Cramer, 2017). Since the upper limbs, especially the hands, play a significant role in daily activity, exploring novel rehabilitation therapies for hand motor recovery in this group is essential (Neumann, 2016). Robot-assisted therapy (RAT) and motor imagery (MI) have been introduced to enhance motor recovery for stroke patients through passive motion or mental practice. However, although these interventions benefit training without requiring patients’ residual ability, rehabilitation effectiveness is still limited by a lack of active engagement (Kwakkel et al., 2008; Ietswaart et al., 2011). Recent advances in brain–computer interface (BCI) technology offer a novel method that could extract the motor intention of patients executing MI to support active rehabilitation training. Related studies have shown promising results that MI-actuated BCI improves motor ability more than pure MI or sham BCI (Ramos-Murguialday et al., 2013; Ang et al., 2014; Pichiorri et al., 2015). However, this intervention still faces limitations in practical use (Mulder, 2007; Baniqued et al., 2021). First, BCI may not be easy for everyone due to the “BCI illiteracy” phenomenon or the limited training schedule in clinical environments (Blankertz et al., 2009; Horowitz et al., 2021). In addition, most stroke subjects show more difficulty executing MI tasks than healthy subjects because of brain impairment in motor-related areas (Mulder, 2007). Worse situations occur in severe patients because they can hardly perform effective MI or fall into fatigue quickly under effortful attempts. Recent studies found that action observation (AO) could also activate sensorimotor features, as in MI and motor execution tasks (Friesen et al., 2017; Hardwick et al., 2017). In addition, repeated AO could induce plasticity changes by activating the mirror neuron system (MNS) (Rizzolatti and Sinigaglia, 2010; Agosta et al., 2017). These inspired studies combined AO in the BCI system, where stronger event-related desynchronization (ERD) responses are found than in pure MI-BCI (Kondo et al., 2015; Ono et al., 2018; Nagai and Tanaka, 2019). However, most of these studies focused on healthy subjects, while related endeavors in the clinical rehabilitation of stroke subjects are still insufficient.
Another major concern in exploring novel interventions in chronic stroke is better evaluating the motor deficits and understanding the therapeutic-induced improvement during rehabilitation neurologically. On the one hand, the recovery in post-stroke motor rehabilitation is usually heterogeneous. Except for individual factors such as age, time since stroke, and related complications, a variety of neuro-clinical factors, such as the degree of brain lesion and neural status, would also affect the patient’s recovery (Riley et al., 2011; Chang et al., 2013; Feng et al., 2015; Kim and Winstein, 2017). On the other hand, chronic stroke recovery is more challenging with the decreasing plasticity of spontaneous recovery and depends more on intervention-induced plasticity (Cassidy and Cramer, 2017). The routinely used assessment of motor recovery is on clinical scales, which are semi-objective and limited in monitoring the underlying neural factors. Hence, recent studies have focused on finding neural biomarkers that could serve as an additional physiological approach to probe brain status and reflect the extent of post-stroke functional recovery (Kim and Winstein, 2017). Potential biomarkers have been found in physiological measuring tools such as Functional magnetic resonance imaging (fMRI) and magnetoencephalograms (MEG) (Várkuti et al., 2013; Kim and Winstein, 2017).
Compared with these tools, electroencephalography (EEG) offers another economical and widely available choice, making it a more practical approach in clinical environments for rehabilitation (Gerloff et al., 2006; Ang and Guan, 2016). In addition, the EEG is easy to implement in EEG-based BCI interventions. However, most related investigations of EEG markers focused on acute or subacute-stage patients, and studies concerned with chronic patients are still lacking (Foreman and Claassen, 2012; Assenza et al., 2017; Trujillo et al., 2017; Bentes et al., 2018). Notably, EEG oscillations in different bands themselves play roles in reflecting the physiological and pathological status of the neural systems. For example, the increasing low-frequency power (delta and theta bands) and decreasing high-frequency power (alpha and beta bands) are believed to reflect the severity of acute neurological deficits (Rabiller et al., 2015; Assenza et al., 2017). Apart from reflecting the motor status, the EEG features may also promote an understanding of varied recovery resulting from additional factors during rehabilitation. For instance, a previous study found that patients under different interventions have different EEG indicators (Mane et al., 2019). We infer that patients with varying degrees of recovery may also differ in EEG features after experiencing different neural processes in training. Overall, how these EEG oscillations would act in chronic stroke and whether related EEG features could reflect therapeutic-induced improvement in effective interventions remains to be determined.
To fill this gap, the present study aimed to explore whether brain oscillations in different EEG bands can reflect the motor status and recovery induced by novel BCI therapy in chronic stroke. Specifically, an AO-BCI robotic hand training intervention was studied in a clinical environment, and the motor scales were assessed before and after the training. The correlations between EEG band power and motor scales both before and after the intervention were analyzed to study their feasibility in reflecting motor status by EEG band power in chronic stroke patients. In addition, we presented the difference in EEG variation during an intervention on patients with and without effective recovery [whether the minimal clinically important difference (MCID) was reached] (van der Lee et al., 2001; Wagner et al., 2008). Moreover, we examined which EEG rhythm variations correlate with motor function improvement and their potential as markers in reflecting therapeutic-induced neuroplasticity changes and guiding rehabilitation intervention in chronic stroke patients. […]

[WEB] Noninvasive method for vagus nerve stimulation shows promise for enhancing motor rehabilitation after stroke
Posted by Kostas Pantremenos in Neuroplasticity, REHABILITATION on July 23, 2023
by Medical University of South Carolina
The longest nerve in the human body starts in the brain and meanders its way down the neck and into the chest, where it splits into separate branches, winding its twisting tendrils to touch each internal organ. Known as the “information superhighway” and aptly named from the Latin word meaning “wanders,” the vagus nerve is a bundle of fibers responsible for the parasympathetic nervous system: digestion, heart rate, breathing.
Sending electrical impulses down this tenth cranial nerve has proven effective in treating conditions like depression and epilepsy, and it has shown great success in amplifying the effects of motor rehabilitation after stroke. Implanting a device onto the vagus nerve in the neck provides direct stimulation to the information superhighway. And using this technology during post-stroke motor rehabilitation was approved by the Food and Drug Administration as a treatment option in 2021.
But researchers at MUSC have found another method that accelerates treatment outcomes and improves motor function after stroke without this invasive and often-uninsured procedure.
As described in a recent paper from Neurorehabilitation and Neural Repair, the research team placed sensors on the upper arm and in the ear and used the connected computer to send timed electrical impulses to the vagus nerve during motor rehabilitation. This noninvasive VNS method, known as motor activated auricular vagus nerve stimulation (MAAVNS), allows patients to gain the same amount of motor function improvement in 4 weeks that patients with the implanted device reached in 6 weeks—all without surgery.
MAAVNS was developed and is currently pending patent at MUSC.
Stroke is a leading cause of disability in the United States according to the American Stroke Association, and it leads to motor deficits and reduced mobility in almost half of stroke survivors over the age of 65. With such a large number affected, Bashar Badran, Ph.D., the director of the Neuro-X lab and Computational Brain Imaging Core at MUSC as well as the principal investigator on the paper, wanted to find a more accessible way to help patients recover.
“Motor rehabilitation is time-consuming and expensive, and oftentimes does not produce the results that patients want,” said Badran. “Technology like the MAAVNS system can boost the effects of conventional motor rehabilitation and help patients get the most out of their therapy in a simple and relatively inexpensive manner. It’s very exciting.”
With a small cart containing the computer and sticker-like sensors, the MAAVNS system can easily be incorporated into occupational therapy settings, with little change or impact to the current standard of care.
With hundreds of repetitions in an hour, patients can focus on specific movements they want to improve, like sewing, cutting fabric or buttoning a shirt, while the MAAVNS system detects when the patient is moving and intricately delivers electrical stimulation to nerves in the ear.
Badran says delivering the stimulation in conjunction with each movement is critical. “Our work shows that outcomes are much better when you time the stimulation in conjuction with movement, in a closed-loop approach,” he said. “Interestingly, more stimulation is not better. In fact, less stimulation timed correctly produces the best outcomes.”
Since the vagus nerve elicits brain activity in areas responsible for the release of neurotransmitters like norepinephrine and serotonin which help the brain learn, stimulating it with electricity facilitates faster learning of motor skills.
While the sample size for this pilot study was small, Badran and his team will be conducting a larger clinical trial next to further study the effects of this noninvasive VNS technique.
“This is really promising technology,” Badran said. “And the fact that the outcomes are mirroring what is already FDA approved is great. Not only do we believe this is an effective new technology for post-stroke motor rehabilitation, but it’s cheaper and easier to incorporate into the standard of care than what is currently available.”
More information: Bashar W. Badran et al, Motor Activated Auricular Vagus Nerve Stimulation as a Potential Neuromodulation Approach for Post-Stroke Motor Rehabilitation: A Pilot Study, Neurorehabilitation and Neural Repair (2023). DOI: 10.1177/15459683231173357
Journal information: Neurorehabilitation and Neural Repair
Provided by Medical University of South Carolina
[WEB] Stroke Recovery: Brain Study Brings Hope For Better Motor Rehabilitation
Posted by Kostas Pantremenos in REHABILITATION on February 7, 2023
RESEARCHERS FROM THE UNIVERSITY OF BIRMINGHAM AND BANGOR UNIVERSITY WHO PUBLISHED THEIR FINDINGS IN THE JOURNAL OF NEUROSCIENCE TODAY BELIEVE THEIR DISCOVERY COULD ENHANCE MOTOR REHABILITATION FOR STROKE SURVIVORS.
New research demonstrates that the human brain prepares expert motions such as playing the piano, participating in sports, or dancing by ‘zipping and unzipping’ information regarding the timing and sequence of movements ahead of the activity being done.
Experts have found that the brain separates the order and time of motions in complicated sequences before zipping and transferring them into individual movement instructions, or “muscle memory,” when the person starts the activity.
They observed that high-level movement sequencing (order and timing) may be maintained across various motor regions of the brain, sometimes across several days of training and memorizing action sequences, before being reactivated by a trigger like a musical cue or a starting gun.
Researchers from Birmingham and Bangor universities published their results today in Journal of Neuroscience, hoping to enhance stroke sufferers’ motor recovery.
Whether it’s writing by hand or playing a musical instrument, the ability to perform movement sequences from memory is a defining characteristic of “skilled human behavior.”
“What is surprising is that,” remarks Principal investigator Dr. Katja Kornysheva, “the brain separates these skills into their constituent features rather than encoding them as an integrated muscle memory, even after extensive training. There is a shift in information states within the brain when performing such tasks.”
When we prepare information for execution, information is obtained from memory unzipped. After that, the information is zipped together, and then the job is started, according to Dr. Kornysheva.
“Perhaps this unzipping mechanism,” according to the researcher, “helps us to stay flexible for adjustments, even in the final hundreds of milliseconds before we start the movement, e.g. if we need to change the speed or timing of an upcoming action.”
In a series of almost a thousand trials, right-handed people (but not professional musicians) learned and memorized four keyboard sequences that they prepared and then played when they saw a cue. Following training, participants typed the keyboard commands in an MRI scanner, which recorded the brain’s activity during the task. In some of the trials, the “go” signal did not occur, which enabled the researchers to distinguish between the act of preparation and the actual action.
“We also found several brain regions which control timing during movement production, but none seemed to control order without integrating it with timing,” points out first author Rhys Yewbrey.
These participants showed a matching effect in their behavior; they learned a sequence with a new order of finger presses more quickly when they were familiar with the timing, but they had difficulty learning a sequence when they had to couple a previously learned order with a new time.
“Perhaps timing control staying active during production allows for flexibility even after the movement has started.”
The brain is thought to distinguish between sequence order and time as “what” aspects denoting higher-level control, which are then combined to determine “exactly” how the job should be carried out.
These new findings contribute to our understanding of how daily abilities like typing, tying shoes, and playing an instrument are stored and regulated in the brain as well as what makes them adaptable and resistant to environmental changes or neurological disorders.
Image Credit: BSIP/Education Images/Universal Images Group via Getty Images
[ARTICLE] Immersive virtual reality for upper limb rehabilitation: comparing hand and controller interaction – Full Text
Posted by Kostas Pantremenos in Paretic Hand, Video Games/Exergames, Virtual reality rehabilitation on December 16, 2022
Abstract
Virtual reality shows great potential as an alternative to traditional therapies for motor rehabilitation given its ability to immerse the user in engaging scenarios that abstract them from medical facilities and tedious rehabilitation exercises. This paper presents a virtual reality application that includes three serious games and that was developed for motor rehabilitation. It uses a standalone headset and the user’s hands without the need for any controller for interaction. Interacting with an immersive virtual reality environment using only natural hand gestures involves an interaction that is similar to that of real life, which would be especially desirable for patients with motor problems. A study involving 28 participants (4 with motor problems) was carried out to compare two types of interaction (hands vs. controllers). All of the participants completed the exercises. No significant differences were found in the number of attempts necessary to complete the games using the two types of interaction. The group that used controllers required less time to complete the exercise. The performance outcomes were independent of the gender and age of the participants. The subjective assessment of the participants with motor problems was not significantly different from the rest of the participants. With regard to the interaction type, the participants mostly preferred the interaction using their hands (78.5%). All four participants with motor problems preferred the hand interaction. These results suggest that the interaction with the user’s hands together with standalone headsets could improve motivation, be well accepted by motor rehabilitation patients, and help to complete exercise therapy at home.
Introduction
More than 17 million people suffer a stroke each year (Krishnamurthi et al. 2013). Due to advances in medicine, stroke mortality has been decreasing, resulting in an increasing number of survivors with motor, psychological, cognitive, social, and economic handicaps that have a negative impact on their quality of life (Lawrence et al. 2001). Six months after a stroke, a large percentage of survivors have motor deficits including hemiparesis (50%) and dependence in activities of daily living (26%) (Go et al. 2013).
At an early stage after stroke, survivors usually have access to rehabilitative care in hospitals, clinics, rehabilitation centers, and other facilities. After those first months, since most patients are medically discharged and do not have the possibility of maintaining treatments, they are encouraged by doctors and therapists to practice exercises at home. However, adherence to exercises that are performed at the patient’s home is usually low, due to lack of motivation, low tolerance for effort, fatigue, or musculoskeletal changes such as joint stiffness or spasticity (Jurkiewicz et al. 2011).
Virtual Reality (VR) with its potential for creating fun and immersive environments and games has emerged as a promising path to increase motivation and encourage survivors to practice motor rehabilitation (Dias et al. 2019; Jonsdottir et al. 2021). This path can replace boring mandatory exercises with entertaining games or activities that are highly customizable to the patient’s own hobbies and tastes. Besides increasing motivation, the use of VR with tracking technologies to monitor gestures will enable the quantification of movements. The use of additional measures for evaluating the general quality of life of patients will, in turn, provide health professionals with the possibility of monitoring the patients’ recovery.
VR has already been successfully used to help patients bear pain and withstand other disease treatments (Schneider and Hood 2007; Patterson et al. 2010; Maani et al. 2011; Baños et al. 2013) as well as to recover from stroke (Cho et al. 2014; Covarrubias et al. 2015). VR offers great potential for rehabilitation (Liu et al. 2016; Laver et al. 2017) since it motivates the patients, allows immersion in engaging virtual environments while providing multiple stimuli, and promotes the improvement of cognitive and motor capacities. Affordable sensors for gesture tracking have been studied and developed (mainly in the gaming industry), which can be explored for rehabilitation (Piron et al. 2009; Covarrubias et al. 2015). This synergy between affordable technology and the benefits it offers makes virtual reality systems tools with great potential for the rehabilitation of stroke, one of the leading causes of disability worldwide.
Telerehabilitation is a promising tool for minimizing the discontinuity of treatment after hospital discharge and for empowering patients to manage their health via interaction with remote rehabilitation professionals (Amorim et al. 2020). VR systems fulfill the fundamental principles of rehabilitation: environments with diversity in stimuli, task-oriented training, intensity, biofeedback, and motivation. All of these are fundamental factors for the success of rehabilitation therapy (Dias et al. 2019). The following benefits of using VR in rehabilitation have already been identified (Laver et al. 2017): increased motivation and collaboration of patients during rehabilitation programs, better performance, neuroplasticity stimulation, improvement of cognitive functions and of the affected limb, and greater autonomy in activities of daily life. Moreover, when combining virtual reality and traditional rehabilitation, stroke patients showed significantly greater improvement in their activities of daily life than those patients treated only with traditional rehabilitation therapy (Kim 2018). This makes VR an interesting tool for therapy. For example, VR therapies have demonstrated to be effective in pain management, in both sick and healthy subjects and have also shown to have very few side effects compared to other more aggressive therapies (Liu et al. 2016). Therefore, VR serious games could be used as a tool to train stroke survivors to monitor their health under the supervision and control of doctors.
The main objective of the work presented here is to develop and test a VR application for upper limb rehabilitation with hand interaction and visualization using a standalone headset in order to identify its strengths and limitations. Three different games were developed mapping simple gestures that are included in Enjalbert’s test (Enjalbert et al. 1988), which is a well-known scale that is used for the functional assessment of the upper limb mobility. We carried out a study to test the developed games regarding performance outcomes and subjective perception with 24 healthy people and 4 people with motor problems. The hypotheses to be corroborated in our study were the following: H1: Users will rate the games positively; H2: There will be no significant differences in the performance of the participants when using controllers or hands; H3: There will be no significant differences in the performance of the participants during the study based on their gender; H4: Participants will express their preference for the use of their hands for interaction. The remainder of this paper is organized as follows: we describe the application and the games developed. For the study, we present and discuss the main results, and finally we draw conclusions and present our ideas for future work. […]

[ARTICLE] Usability of the REHOME Solution for the Telerehabilitation in Neurological Diseases: Preliminary Results on Motor and Cognitive Platforms – Full Text
Posted by Kostas Pantremenos in Cognitive Rehabilitation, Tele/Home Rehabilitation, Video Games/Exergames on December 10, 2022
Abstract
The progressive aging of the population and the consequent growth of individuals with neurological diseases and related chronic disabilities, will lead to a general increase in the costs and resources needed to ensure treatment and care services. In this scenario, telemedicine and e-health solutions, including remote monitoring and rehabilitation, are attracting increasing interest as tools to ensure the sustainability of the healthcare system or, at least, to support the burden for health care facilities. Technological advances in recent decades have fostered the development of dedicated and innovative Information and Communication Technology (ICT) based solutions, with the aim of complementing traditional care and treatment services through telemedicine applications that support new patient and disease management strategies. This is the background for the REHOME project, whose technological solution, presented in this paper, integrates innovative methodologies and devices for remote monitoring and rehabilitation of cognitive, motor, and sleep disorders associated with neurological diseases. One of the primary goals of the project is to meet the needs of patients and clinicians, by ensuring continuity of treatment from healthcare facilities to the patient’s home. To this end, it is important to ensure the usability of the solution by elderly and pathological individuals. Preliminary results of usability and user experience questionnaires on 70 subjects recruited in three experimental trials are presented here.
1. Introduction
In recent years we have been witnessing a gradual increase in life expectancy, due to the general improvement in lifestyles and advances in medicine. This phenomenon has led to the progressive aging of the population, with significant consequences for future economic and social policies [1]. Reports on aging by the World Health Organization (WHO) confirm this trend, further indicating that the population over 65 will double and the population over 80 will triple by 2050 [2]. In addition, several studies and reports on global health have highlighted that aging is inherently accompanied by an increase in the number of people with age-related diseases and disabilities [3,4] that need specific health care treatments for prolonged periods. The same reports also show that neurological diseases and acute events such as stroke, which have a prevalence in the older population, are already very common in industrialized countries characterized by greater well-being. Neurological and neurodegenerative diseases, such as dementia and Parkinson’s disease, have a dramatic impact on quality of life since they progressively induce severe and chronic disabilities in the cognitive and motor domains. Stroke is a major cause of comorbidity and disability [5], in which hemiplegia (or hemiparesis) and impaired gait are common consequences of the loss of brain function in cortical motor areas. Parkinson’s disease is recognized as the second most common neurodegenerative disorder after Alzheimer’s disease, and it causes a progressive impairment in motor functions (bradykinesia). In addition, sleep disorders are common comorbidities in the neurological and post-stroke clinical picture that require complex instrumental investigations and specific treatment to avoid consequences in daily activities [6].
This scenario thus imposes significant efforts and challenges for prolonged patient care, and in particular, rehabilitation programs that aim to mitigate the adverse effects and still ensure the best quality of life [7,8,9]. Several studies have also shown that rehabilitation outcomes are better when patients can continue rehabilitation treatment at home [10]. Moreover, it is clear that the health sector will be one of the most affected by the demographic change in the coming years, in terms of the sustainability of health services [11].
To address future challenges in healthcare, solutions based on Information and Communication Technology (ICT) [12] are attracting increasing interest in finding a trade-off between patient needs (quality of life), effectiveness of healthcare services (monitoring and rehabilitation protocols), and sustainability of the healthcare system (cost and resources). For example, ICT solutions could support new patient and disease management strategies based on telemedicine and related applications, thereby moving monitoring and rehabilitation services from health care facilities to home settings [13,14], thus favoring continuous and remote follow-up of patients. However, there are still several barriers that limit its deployment in the home environment, including aspects of usability, acceptability, lack of motivation and skepticism in using digital tools, especially in the elderly and individuals with severe chronic disabilities [10,15,16].
This is the background for the REHOME project [17], whose technological solution is presented in this paper. The project involved twelve partners (including seven small and medium-sized enterprises, three research institutions, and two hospitals) and was developed in a multidisciplinary context of technological and clinical expertise to achieve a helpful, comprehensive, and integrated home solution. The project focuses on the remote monitoring and rehabilitation of cognitive, motor, and sleep disorders originating from neurological diseases and injuries, in particular stroke, mild cognitive impairment, and Parkinson’s disease. To this end, the proposed solution integrates innovative technologies and methodologies able to ensure patient engagement and continuity of care, monitoring, and rehabilitation in supervised and minimally supervised scenarios. The REHOME solution exploits different types of sensors and devices (such as optical sensors, electromyography, commercial and prototypal sensors for physiological signals); innovative methods for rehabilitation (such as virtual reality, exergaming, gamification techniques); traditional and gamified motor tasks, derived from standardized clinical scales, to evaluate patients’ current condition; estimation of objective features to quantify the patients’ performance and progression over time. At the same time, it facilitates communication and interaction between doctor, patient, and caregiver through the infrastructure of the healthcare platform and its web-based facilities.
In recent years, home-based rehabilitation has been widely considered and many solutions have been developed for this purpose, as evidenced by some recent literature reviews. The study by Hosseiniravandi et al. [18] compared twenty-two solutions for remote rehabilitation focused primarily on five categories of diseases and disorders, including musculoskeletal, neurological, respiratory, cardiovascular, and other general health-related problems. The analysis revealed that these solutions shared three common functionalities, namely exercise plan management, outcome reporting, and patient education. There were also many similarities in terms of methods to collect data: automatic data collection (85%), recording of patient treatment progress (90%), and providing periodic (75%) and real-time (85%) feedback to therapists and patients. In contrast, the study did not focus on the hardware components and devices used in the solutions analyzed, nor on the real-world implemented systems, infrastructure, and the effects of these systems in real world settings. The study by de Souza et al. [19] examined fourteen home-based rehabilitation frameworks based on gaming or exergaming approaches. The findings of the study showed that most of the solutions (79%) focused on stroke, motor, and cognitive rehabilitation. In addition, only 22% of them were cloud-based applications. In [20], a detailed overview of telerehabilitation and its fields of application was provided, with an analysis of the benefits and drawbacks associated with its use. The study highlighted the main disadvantages of telerehabilitation, including patient skepticism due to remote interaction with therapists. The study also suggested that further research is needed to improve electronic equipment and devices, and to make applications as flexible as possible to increase the reliability and effectiveness of telerehabilitation equipment for treating patients with specific problems. The effectiveness of telerehabilitation was investigated in post-stroke subjects and individuals with mild cognitive impairment in [21,22], respectively. Both studies concluded that telerehabilitation can be an appropriate alternative to the usual rehabilitation care.
However, as also pointed out by the previously mentioned state-of-the-art studies, several challenges remain [15,23]. For example, patient satisfaction, patient involvement, and acceptability of the proposed remote rehabilitation approach are generally given little consideration. The same occurs for physical discomfort caused by sensors and devices, which are often invasive or complex to use. In most cases, a single methodological approach is provided that is not suitable for different therapeutic areas, pathological conditions, usage scenario, and current patient status. In addition, a proper user interface design, which is critical especially in the case of people with disabilities, is often neglected. Finally, the lack of adequate training on the use of technological devices and solutions is another common weakness that often prevents patients from using them effectively and continuing the planned therapeutic treatment.
The REHOME project, and the implemented solution, addressed some of the weaknesses highlighted, such as increasing patient involvement; understanding and addressing the specific and necessary functions for the different target pathologies (each with its own peculiarities); managing the personalized treatment plan and evaluating the effectiveness of remote treatment; addressing aspects related to usability, taking care of the user interface, interaction methods, and the simplicity of the devices involved; integrating multiple sensors and methodological approaches to evaluate different domains (motor, cognitive, and sleep) but common to the target pathologies. All these features represent the innovative and peculiar aspect of the proposed solution with respect to the state-of-the-art.
Currently, the project is in its final experimental phase at hospitals. However, some pilot trials were previously organized with the aim of obtaining feedback on the usability of some tools included in the implemented solution. The main objective of the paper is to present the preliminary results related to usability, one of the key elements is to propose a suitable and helpful solution for remote monitoring and rehabilitation. Specifically, the contributions of this work concern the following points:
- Introducing the technological solution developed in the REHOME project, highlighting its main components, innovative features, and methodological approaches to meet the needs of patients and healthcare professionals and overcome the main weaknesses of telemedicine and eHealth services that emerge in the literature;
- Presenting three experimental protocols concerning the motor and cognitive platforms that involved groups of elderly patients affected by Parkinson’s disease (and forms of atypical parkinsonism) and mild cognitive impairment, target pathologies of the REHOME project;
- Presenting the preliminary results on the usability and user experience evaluation using questionnaires administered to the participants to get feedback on the strengths and weaknesses of the developed platforms.
Indeed, it should be noted that this paper is an extended and more detailed version of the one recently presented at the second IEEE Conference on ICT solutions for eHealth (ICTS4eHealth 2022) [24].
The next sections are organized as follows: Section 2 presents the implemented solution, describing the overall architecture and its main components, with a focus on those considered for usability evaluation; Section 3 presents the main results on the usability questionnaires according to the organized pilot trials; Section 4 contains discussion and future developments; Section 5 contains some concluding remarks.
2. Materials and Methods
2.1. The Architecture of the Solution
REHOME is a tele-rehabilitation system for personalized and gamified patient training and monitoring by Healthcare Professionals (HCPs). Patients at home can carry out exercises according to their own rehabilitation plan by using the enabling device kit provided by the doctor. Rehabilitation and health evaluation session data are then collected to allow remote and timely analysis and, if deemed necessary, improvements to the plan.
As depicted in Figure 1, the REHOME system, developed with cloud technologies and based on a distributed microservices architecture, is composed of several subsystems:
- HCP Platform (HCPP): to monitor patients remotely and to assess their progress;
- Cognitive Rehabilitation and Gaming Platform (CRGP): based on gaming to train five different cognitive domains and to improve memory and orientation skills;
- Motor Rehabilitation and Exergames Platform (MREP): for automatic assessment and rehabilitation of motor disabilities concerning limbs, posture, balance, and coordination;
- Sleep Evaluation Platform (SEP): to detect and evaluate sleep disorders.
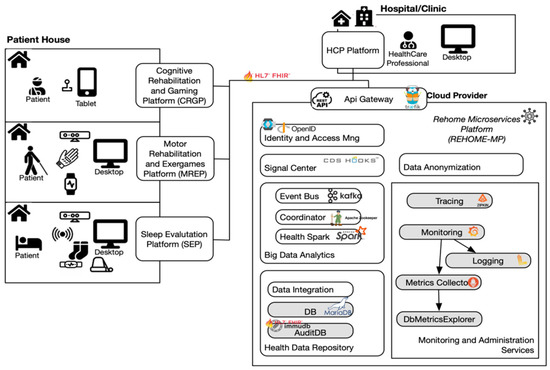
Figure 1. REHOME system architecture.
[ARTICLE] The effect of gamified robot-enhanced training on motor performance in chronic stroke survivors – Full Text
Posted by Kostas Pantremenos in Paretic Hand, Rehabilitation robotics, Video Games/Exergames on November 27, 2022
Abstract
Task-specific training constitutes a core element for evidence-based rehabilitation strategies targeted at improving upper extremity activity after stroke. Its combination with additional treatment strategies and neurotechnology-based solutions could further improve patients’ outcomes. Here, we studied the effect of gamified robot-assisted upper limb motor training on motor performance, skill learning, and transfer with respect to a non-gamified control condition with a group of chronic stroke survivors. The results suggest that a gamified training strategy results in more controlled motor performance during the training phase, which is characterized by a higher accuracy (lower deviance), higher smoothness (lower jerk), but slower speed. The responder analyses indicated that mildly impaired patients benefited most from the gamification approach. In conclusion, gamified robot-assisted motor training, which is personalized to the individual capabilities of a patient, constitutes a promising investigational strategy for further improving motor performance after a stroke.
1. Introduction
Stroke is a major contributor to the global burden of disease [1]. It has been estimated that the absolute number of stroke survivors remaining disabled after an ischemic stroke has increased 1.4- to 1.8-folds between 1990 and 2013 [1]. Particularly, many stroke survivors are affected by upper limb motor impairment, in which magnitude largely determines the successful reintegration into an independent personal and professional life [2, 3]. This current state urgently requires further development of efficient treatments for post-stroke motor rehabilitation.
Currently, task-specific training constitutes a core element of evidence-based upper extremity rehabilitation programs [4]. Task-specific training relies on the finding that repetitive and consistent practice of meaningful and challenging tasks optimally engage intrinsic neuronal plasticity and can result in meaningful functional improvements [5]. Task-specific training can be incorporated or combined with other emerging behavioral interventions such as constraint-induced movement therapy, mirror therapy, motor imagery/mental practice [6] and neurotechnology-based solutions such as robot-assisted training [4, 7, 8]. Recently, a set of research strategies has been proposed aiming to further facilitate the design of efficient novel rehabilitation approaches and their clinical implementation [3]. Some of them are: (i) the combined application, (ii) personalization, and (iii) intensified training.
In our present work, we investigated the combined application of a gamified training strategy and robot-assisted upper extremity training. Gamification is defined as the use of game design elements in traditionally non-game contexts [9]. Common game design elements are, for instance, specific tasks (e.g., collect a target), rules (e.g., do not crash into the walls of a maze), or point systems (e.g., number of collected targets) [10]. Researchers have proposed that when designing gamified applications for rehabilitation, two game design principles are of particular importance – meaningful play and challenge [11]. Meaningful play corresponds to the presence of an apparent relationship between own actions and the system outcome for the user [12]. Challenge refers to the optimal adaptation of the task demands to the user’s ability accounting for the trade-off of being potentially too easy and thereby risking loss of interest and boredom or of being too difficult, which may lead to frustration and termination of the activity [11]. Both design principles were incorporated into our tested intervention. The motor training was implemented using the Cellulo robotic platform [14, 15]. The platform consists of palm-sized, graspable, haptic-enabled tangible robots, printed paper sheets on which the robots are operating and a tablet/phone or computer controlling the application [13]. These “computer-mouse-like” robots allow users to interact with printed visual elements on paper, such as walls in a maze, and can provide visual and haptic feedback. Our main aim by using the Cellulo platform was to provide an intuitive, easy-to-use and easy-to-set-up system for motor training that allows for tangible interaction with the game elements.
Previous research has tested the use of gamification strategies for rehabilitation applications, for review see, e.g., Ferreira and colleagues [10]. However, knowledge on the induced behavioral pattern and possible underlying mechanisms in clinical populations is largely lacking. In this proof-of-principle study, we strove to address this by systematically testing potential effects of a gamified robot-based upper extremity motor training in a controlled study. We hypothesized that a gamified application strategy leads to enhanced control of the robotic device during the training sessions when compared to a non-gamified control condition resulting in enhanced motor performance. Please see Figure 1 for an illustration of an exemplary scenes while a patient trains with both modalities. The respective experimental work and analyses were guided by the following research questions:•
RQ1: Does a gamified application lead to enhanced motor performance during the training phase compared to a non-gamified application?•
RQ2: Does a gamified training application lead to enhanced motor skill learning compared to a non-gamified application?•
RQ3: Does the training strategy – gamified versus non-gamified – have an impact on skill transfer to simple robot manipulation?•
RQ4: Is the magnitude of the gamification effect associated with patient characteristics determined by clinical motor scales and assessments?•
RQ5: Which training strategy do patients prefer based on their self-assessment?

In the following sections, we characterize the induced behavioral pattern and provide insights into potential underlying mechanisms of gamified robot-assisted motor training paradigms allowing one to further test the potential of haptic-enabled robot-enhanced gamified training in future randomized clinical trials. […]
[Abstract + References] MoveLeg: An Assistive Device for the Motor Tele-Rehabilitation of the Lower Limbs – Book Chapter
Posted by Kostas Pantremenos in Gait Rehabilitation - Foot Drop, Tele/Home Rehabilitation on November 24, 2022
Abstract
The use of assistive devices has shown to be beneficial in clinical services for the motor rehabilitation of the lower limbs. Its use facilitates the rehabilitation of patients and increases their participation in daily life. However, unlike some countries like the USA, Japan and Germany, in practice, these technologies are not necessarily available in Latin American countries due to a lack of resources, and demographic access barriers. In this work we present the design, development, and preliminary evaluation in terms of functionality and usability of MoveLeg, a low-cost assistive device for the motor rehabilitation of the lower limbs of patients with sequelae of stroke. The proposed device is based on design insights obtained from 14 motor rehabilitation specialists from different institutions, with characteristics that allow to:
(i) care for remote patients,
(ii) manage the therapy program online,
(iii) personalize and monitor each session based on a mirror therapy, and
(iv) decrease the intensity or stop the exercise in real-time.
The results of the preliminary functional evaluation establish that the device is working properly. In addition, the results of the usability evaluation with an older adult and a physiotherapist suggest that the device is perceived as easy to use, easy to learn, and with a high intention to use, while providing motor tele-rehabilitation in real-time, in a supervised and safe way.
References
- WHO, UNICEF Global report on assistive technology (2022)Google Scholar
- Demain, S., Burridge, J., Ellis-Hill, C., et al.: Assistive technologies after stroke: self-management or fending for yourself? A focus group study. BMC Health Serv. Res. 13(1), 1–12 (2013)CrossRef Google Scholar
- Goldstein, L.B., Bushnell, C.D., Adams, R.J., et al.: guidelines for the primary prevention of stroke: a guideline for healthcare professionals from the American heart association/American stroke association. Stroke 42, 517–584 (2011)CrossRef Google Scholar
- Albert, S.J., Kesselring, J.: Neurorehabilitation of stroke. J. Neurol. 259, 817–832 (2012). https://doi.org/10.1007/s00415-011-6247-yCrossRef Google Scholar
- Gandhi, D., Sterba, A., Khatter, H., Pandian, J.D.: Mirror therapy in stroke rehabilitation: current perspectives. Ther. Clin. Risk Manag. 16, 75–85 (2020)CrossRef Google Scholar
- Meng, W., Liu, Q., Zhou, Z., et al.: Recent development of mechanisms and control strategies for robot-assisted lower limb rehabilitation. Mechatronics 31, 132–145 (2015)CrossRef Google Scholar
- MacLachlan, M., Scherer, M.: Systems thinking for assistive technology: a commentary on the GREAT summit. Disabil. Rehabil. Assist. Technol. 13, 492–496 (2018)CrossRef Google Scholar
- WHO.: Rehabilitation in Health Systems (2017)Google Scholar
- Ruiz-Zafra, Á., Benghazi, K., Noguera, M., Garrido, J.: Zappa: an open mobile platform to build cloud-Based m-health systems. In: Advances in Intelligent Systems and Computing, pp 87–94 (2013)Google Scholar
- Ruiz-Zafra, A., Noguera, M., Benghazi, K., Garrido, J.L., Urbano, G.C., Caracuel, A.: Cloud and web services integration for mHealth telerehabilitation support. In: Canal, C., Villari, M. (eds.) ESOCC 2013. CCIS, vol. 393, pp. 266–276. Springer, Heidelberg (2013). https://doi.org/10.1007/978-3-642-45364-9_22CrossRef Google Scholar
- Okada, S., Sakaki, T., Hirata, R., et al.: TEM: a therapeutic exercise machine for the lower extremities of spastic patients. Adv. Robot. 14, 597–606 (2000)CrossRef Google Scholar
- Girone, M., Burdea, G., Bouzit, M., et al.: A Stewart Platform-Based System for Ankle Telerehabilitation. In: Proceedings of the ASME, Dynamic Systems and Control Division, pp 203–212 (2001)Google Scholar
- Saglia, J., Tsagarakis, N., Dai, J., Caldwell, D.: A high-performance redundantly actuated parallel mechanism for ankle rehabilitation. Int. J. Rob. Res. 28, 1216–1227 (2009)CrossRef Google Scholar
- Xie, S., Jamwal, P.: An iterative fuzzy controller for pneumatic muscle driven rehabilitation robot. Expert Syst. Appl. 38, 8128–8137 (2011)CrossRef Google Scholar
- Bradley, D., Acosta-Marquez, C., Hawley, M., et al.: NeXOS-The design, development and evaluation of a rehabilitation system for the lower limbs. Mechatronics 19, 247–257 (2009)CrossRef Google Scholar
- Akdoǧan, E., Adli, M.: The design and control of a therapeutic exercise robot for lower limb rehabilitation: Physiotherabot. Mechatronics 21, 509–522 (2011)CrossRef Google Scholar
- Kinetec Spectra Knee CPM Machine. https://www.kinetecusa.com/kinetec-spectra-with-washable-pads. Accessed 25 June 2021
- Kinetec Breva Ankle/Foot CPM. https://www.kinetecusa.com/kinetec-breva. Accessed 31 Oct 2017
- CPM 4060. http://www.innovamedica.com.ec/productos-carci-biomecanica.html. Accessed 15 Sep 2018
- CPMotion. https://www.btlnet.es/productos-fisioterapia-cpmotion. Accesed 28 Feb 2016
- Knee CPM. https://medicalpremium.com.mx/producto/movilizador-rodilla-renta-terapia/. Accessed 30 June 2016
- Knee CPM. https://medicalpremium.com.mx/producto/movilizador-rodillas/. Accessed 25 Feb 2017
- Avila-Chaurand, R., Prado-León, L., González-Muñoz, E.: Dimensiones antropométricas de población latinoamericana. Universidad de Guadalajara (2007)Google Scholar
- Brooke, J.: SUS – A quick and dirty usability scale. Usability Eval. Ind. 189(194), 4–7 (1996)Google Scholar
- Davis, F.: Perceived usefulness, perceived ease of use, and user acceptance of information technology. MIS Q. 13, 319–340 (1989)CrossRef Google Scholar
[Abstract] Basis and Clinical Evidence of Virtual Reality-Based Rehabilitation of Sensorimotor Impairments After Stroke – BOOK Chapter
Posted by Kostas Pantremenos in Books, Virtual reality rehabilitation on November 19, 2022
Abstract
In the recent years, the use of virtual reality (VR) to enhance motor skills of persons with activity and participation restriction due to disease or injury has become an important area of research and translation to practice. In this chapter, we describe the design of such VR systems and their underlying principles, such as experience-dependent neuroplasticity and motor learning. Further, psychological constructs related to motivation, including salience, goal setting, and rewards are commonly utilized in VR to optimize motivation during rehabilitation activities. Hence, virtually simulated activities are considered to be ideal for [1] the delivery of specific feedback, [2] the ability to perform large volumes of training, and [3] the presentation of precisely calibrated difficulty levels, which maintain a high level of challenge throughout long training sessions. These underlying principles are contrasted with a growing body of research comparing the efficacy of VR with traditionally presented rehabilitation activities in persons with stroke that demonstrate comparable or better outcomes for VR. In addition, a small body of literature has utilized direct assays of neuroplasticity to evaluate the effects of virtual rehabilitation interventions in persons with stroke. Promising developments and findings also arise from the use of off-the-shelf video game systems for virtual rehabilitation purposes and the integration of VR with robots and brain-computer interfaces. Several challenges limiting the translation of virtual rehabilitation into routine rehabilitation practice need to be addressed but the field continues to hold promise to answer key issues faced by modern healthcare.
References
Burdea GC, Coiffet P. Virtual reality technology. Presence. 2003;12(6):663–4.
Wilson PN, Foreman N, Stanton D. Virtual reality, disability and rehabilitation. Disabil Rehabil. 1997;19(6):213–20.
CrossRef CAS PubMed Google Scholar
Adamovich SV, et al. Sensorimotor training in virtual reality: a review. NeuroRehabil. 2009;25(1):29–44.
Slater M. Place illusion and plausibility can lead to realistic behaviour in immersive virtual environments. Philos Trans R Soc B Biol Sci. 2009;364(1535):3549–57.
Slater M, Wilbur S. A framework for immersive virtual environments (FIVE): Speculations on the role of presence in virtual environments. Presence. 1997;6(6):603–16.
Baños RM, et al. Immersion and emotion: their impact on the sense of presence. Cyberpsychol Behav. 2004;7(6):734–41.
CrossRef PubMed Google Scholar
Llorens R, et al. Tracking systems for virtual rehabilitation: objective performance vs subjective experience: a practical scenario. Sensors. 2015;15(3):6586–606.
CrossRef PubMed PubMed Central Google Scholar
Stanney KM, Mourant RR, Kennedy RS. Human factors issues in virtual environments: a review of the literature. Presence. 1998;7(4):327–51.
Arzy S, et al. Neural mechanisms of embodiment: asomatognosia due to premotor cortex damage. Arch Neurol. 2006;63(7):1022–5.
CrossRef PubMed Google Scholar
Legrand D. The bodily self: the sensori-motor roots of pre-reflective self-consciousness. Phenom Cog Sci. 2006;5(1):89–118.
For more Download references
