Introduction
Worldwide, stroke is a leading cause of adult long-term disability (Mozaffarian et al., 2015). From those who survive, an increased number is suffering with severe cognitive and motor impairments, resulting in loss of independence in their daily life such as self-care tasks and participation in social activities (Miller et al., 2010). Rehabilitation following stroke is a multidisciplinary approach to disability which focuses on recovery of independence. There is increasing evidence that chronic stoke patients maintain brain plasticity, meaning that there is still potential for additional recovery (Page et al., 2004). Traditional motor rehabilitation is applied through physical therapy and/or occupational therapy. Current approaches of motor rehabilitation include functional training, strengthening exercises, and range of movement exercises. In addition, techniques based on postural control, stages of motor learning, and movement patterns have been proposed such as in the Bobath concept and Bunnstrom approach (amongst others) (Bobath, 1990). After patients complete subacute rehabilitation programs, many still show significant upper limb motor impairment. This has important functional implications that ultimately reduce their quality of life. Therefore, alternative methods to maximize brain plasticity after stroke need to be developed.
So far, there is growing evidence that action observation (AO) (Celnik et al., 2008) and motor imagery (MI) improve motor function (Mizuguchi and Kanosue, 2017) but techniques based on this paradigm are not widespread in clinical settings. As motor recovery is a learning process, the potential of MI as a training paradigm relies on the availability of an efficient feedback system. To date, a number of studies have demonstrated the positive impact of virtual-reality (VR) based on neuroscientific grounds on recovery, with proven effectiveness in the stroke population (Bermúdez i Badia et al., 2016). However, patients with no active movement cannot benefit from current VR tools due to low range of motion, pain, fatigue, etc. (Trompetto et al., 2014). Consequently, the idea of directly training the central nervous system was promoted by establishing an alternative pathway between the user’s brain and a computer system.
This is possible by using electroencephalography (EEG)-based Brain-Computer Interfaces (BCIs), since they can provide an alternative non-muscular channel for communication and control to the external world (Wolpaw et al., 2002), while they could also provide a cost-effective solution for training (Vourvopoulos and Bermúdez, 2016b). In rehabilitation, BCIs could offer a unique tool for rehabilitation since they can stimulate neural networks through the activation of mirror neurons (Rizzolatti and Craighero, 2004) by means of action-observation (Kim et al., 2016), motor-intent and motor-imagery (Neuper et al., 2009), that could potentially lead to post-stroke motor recovery. Thus, BCIs could provide a backdoor to the activation of motor neural circuits that are not stimulated through traditional rehabilitation techniques.
In EEG-based BCI systems for motor rehabilitation, Alpha (8–12 Hz) and Beta (12–30 Hz) EEG rhythms are utilized since they are related to motor planning and execution (McFarland et al., 2000). During a motor attempt or motor imagery, the temporal pattern of the Alpha rhythms desynchronizes. This rhythm is also named Rolandic Mu-rhythm or the sensorimotor rhythm (SMR) because of its localization over the sensorimotor cortices. Mu-rhythms are considered indirect indications of functioning of the mirror neuron system and general sensorimotor activity (Kropotov, 2016). These are often detected together with Beta rhythm changes in the form of an event-related desynchronization (ERD) when a motor action is executed (Pfurtscheller and Lopes da Silva, 1999). These EEG patterns are primarily detected during task-based EEG (e.g., when the participant is actively moving or imagining movement) and they are of high importance in MI-BCIs for motor rehabilitation.
A meta-analysis of nine studies (combined N = 235, sample size variation 14 to 47) evaluated the clinical effectiveness of BCI-based rehabilitation of patients with post-stroke hemiparesis/hemiplegia and concluded that BCI technology could be effective compared to conventional treatment (Cervera et al., 2018). This included ischemic and hemorrhagic stroke in both subacute and chronic stages of stoke, between 2 to 8 weeks. Moreover, there is evidence that BCI-based rehabilitation promotes long-lasting improvements in motor function of chronic stroke patients with severe paresis (Ramos-Murguialday et al., 2019), while overall BCI’s are starting to prove their efficacy as rehabilitative technologies in patients with severe motor impairments (Chaudhary et al., 2016).
The feedback modalities used for BCI motor rehabilitation include: non-embodied simple two-dimensional tariffs on a screen (Prasad et al., 2010; Mihara et al., 2013), embodied avatar representation of the patient on a screen or with augmented reality (Holper et al., 2010; Pichiorri et al., 2015), neuromuscular electrical stimulation (NMES) (Kim et al., 2016; Biasiucci et al., 2018). and robotic exoskeletal orthotic movement facilitation (Ramos-Murguialday et al., 2013; Várkuti et al., 2013; Ang et al., 2015). In addition, it has been shown that multimodal feedback lead to a significantly better performance in motor-imagery (Sollfrank et al., 2016) but also multimodal feedback combined with motor-priming, (Vourvopoulos and Bermúdez, 2016a). However, there is no evidence which modalities are more efficient in stroke rehabilitation are.
Taking into account all previous findings in the effects of multimodal feedback in MI training, the purpose of this case study is to examine the effect of the MI paradigm as a treatment for post-stroke upper limb motor dysfunction using the NeuRow BCI-VR system. This is achieved through the acquisition of clinical scales, dynamics of EEG during the BCI treatment, and brain activation as measured by functional MRI (fMRI). NeuRow is an immersive VR environment for MI-BCI training that uses an embodied avatar representation of the patient arms and haptic feedback. The combination of MI-BCIs with VR can reinforce activation of motor brain areas, by promoting the illusion of physical movement and the sense of embodiment in VR (Slater, 2017), and hence further engaging specific neural networks and mobilizing the desired neuroplastic changes. Virtual representation of body parts paves the way to include action observation during treatment. Moreover, haptic feedback is added since a combination of feedback modalities could prove to be more effective in terms of motor-learning (Sigrist et al., 2013). Therefore, the target of this system is to be used by patients with low or no levels of motor control. With this integrated BCI-VR approach, severe cases of stroke survivors may be admitted to a VR rehabilitation program, complementing traditional treatment.
Methodology
Patient Profile
In this pilot study we recruited a 60 years old male patient with left hemiparesis following cerebral infarct in the right temporoparietal region 10 months before. The participant had corrected vision through eyewear, he had 4 years of schooling and his experience with computers was reported as low. Moreover, the patient was on a low dose of diazepam (5 mg at night to help sleep), dual antiplatelet therapy, anti-hypertensive drug and metformin. Hemiparesis was associated with reduced dexterity and fine motor function; however, sensitivity was not affected. Other sequelae of the stroke included hemiparetic gait and dysarthria. Moreover, a mild cognitive impairment was identified which did not interfere with his ability to perform the BCI-VR training. The patient had no other relevant comorbidities. Finally, the patient was undergoing physiotherapy and occupational therapy at the time of recruitment and had been treated with botulinum toxin infiltration 2 months before due to focal spasticity of the biceps brachii.
Intervention Protocol
The patient underwent a 3-weeks intervention with NeuRow, resulting in 10 BCI sessions of a 15 min of exposure in VR training per session. Clinical scales, motor imagery capability assessment, and functional -together with structural- MRI data had been gathered in three time-periods: (1) before (serving as baseline), (2) shortly after the intervention and (3) one-month after the intervention (to assess the presence of long-term changes). Finally, electroencephalographic (EEG) data had been gathered during all sessions, resulting in more than 20 datasets of brain electrical activity.
The experimental protocol was designed in collaboration with the local healthcare system of Madeira, Portugal (SESARAM) and approved by the scientific and ethic committees of the Central Hospital of Funchal. Finally, written informed consent was obtained from the participant upon recruitment for participating to the study but also for the publication of the case report in accordance with the 1964 Declaration of Helsinki.
Assessment Tools
A set of clinical scales were acquired including the following:
1. Montreal Cognitive Assessment (MoCA). MoCA is a cognitive screening tool, with a score range between 0 and 30 (a score greater than 26 is considered to be normal) validated also for the Portuguese population, (Nasreddine et al., 2005).
2. Modified Ashworth scale (MAS). MAS is a 6-point rating scale for measuring spasticity. The score range is 0, 1, 1+, 2, 3, and 4 (Ansari et al., 2008).
3. Fugl-Meyer Assessment (FMA). FMA is a stroke specific scale that assesses motor function, sensation, balance, joint range of motion and joint pain. The motor domain for the upper limb has a maximum score of 66 (Fugl-Meyer et al., 1975).
4. Stroke Impact Scale (SIS). SIS is a subjective scale of the perceived stroke impact and recovery as reported by the patient, validated for the Portuguese population. The score of each domain of the questionnaire ranges from 0 to 100 (Duncan et al., 1999).
5. Vividness of Movement Imagery Questionnaire (VMIQ2). VMIQ2 is an instrument that assess the capability of the participant to perform imagined movements from external perspective (EVI), internal perspective imagined movements (IVI) and finally, kinesthetic imagery (KI) (Roberts et al., 2008).
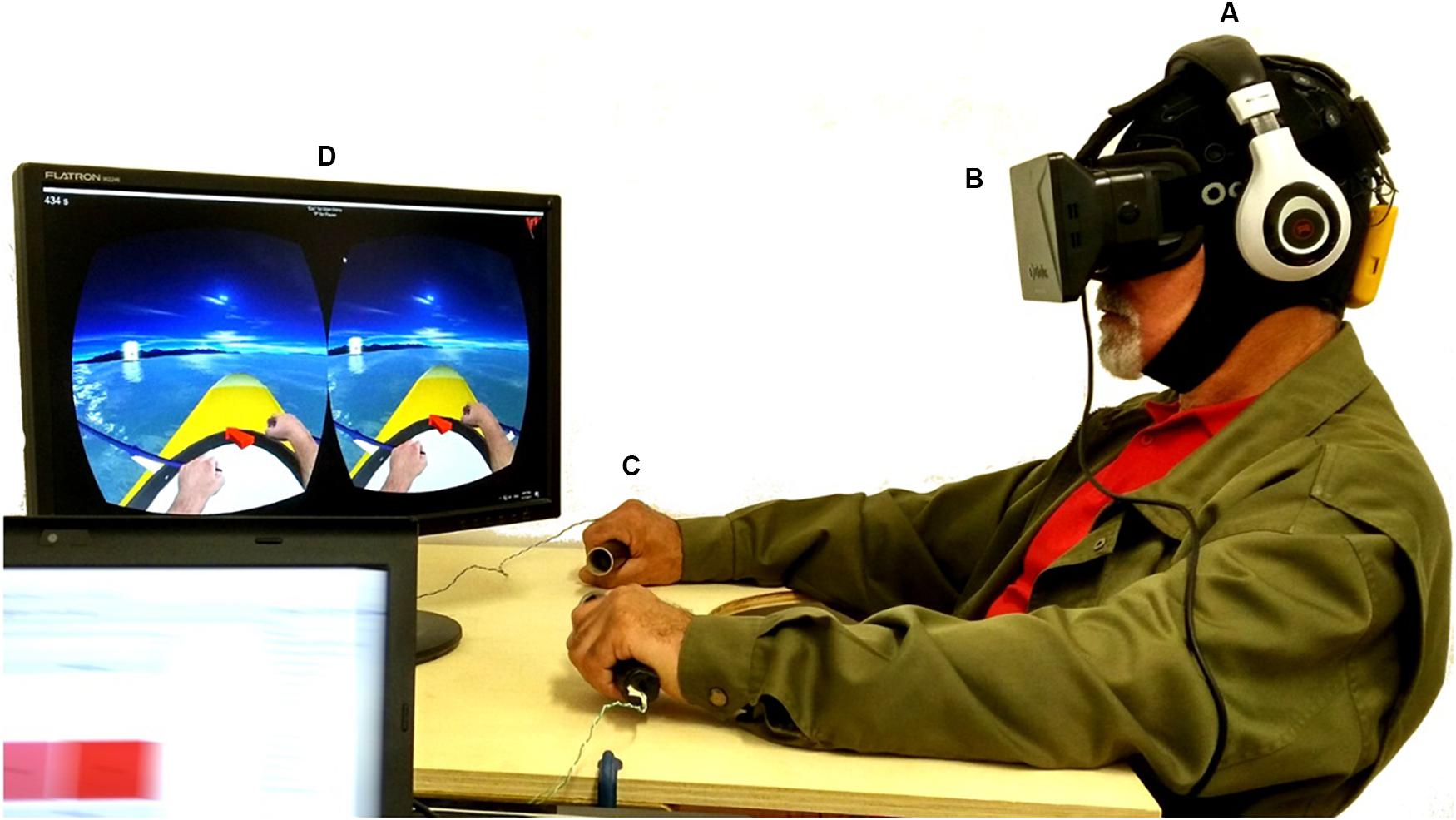
NeuRow BCI-VR System
EEG Acquisition
For EEG data acquisition, the Enobio 8 (Neuroelectrics, Barcelona, Spain) system was used. Enobio is a wearable wireless EEG sensor with 8 EEG channels for the recording and visualization of 24-bit EEG data at 500 Hz and a triaxial accelerometer. The spatial distribution of the electrodes followed the 10–20 system configuration (Klem et al., 1999) with the following electrodes over the somatosensory and motor areas: Frontal-Central (FC5, FC6), Central (C1, C2, C3, C4), and Central-Parietal (CP5, CP6) (Figure 1A). The EEG system was connected via Bluetooth to a dedicated desktop computer, responsible for the EEG signal processing and classification, streaming the data via UDP through the Reh@Panel (RehabNet Control Panel) for controlling the virtual environment. The Reh@Panel is a free tool that acts as a middleware between multiple interfaces and virtual environments (Vourvopoulos et al., 2013).