ABSTRACT
Upper extremity weakness in chronic stroke remains a problem not fully addressed by current therapies. Brain–computer interfaces (BCIs) engaging the unaffected hemisphere are a promising therapy that are entering clinical application, but the mechanism underlying recovery is not well understood. We used resting state functional MRI to assess the impact a contralesionally driven EEG BCI therapy had on motor system functional organization. Patients used a therapeutic BCI for 12 weeks at home. We acquired resting-state fMRI scans and motor function data before and after the therapy period. Changes in functional connectivity (FC) strength between motor network regions of interest (ROIs) and the topographic extent of FC to specific ROIs were analyzed. Most patients achieved clinically significant improvement. Motor FC strength and topographic extent decreased following BCI therapy. Motor recovery correlated with reductions in motor FC strength across the entire motor network. These findings suggest BCI-mediated interventions may reverse pathologic strengthening of dysfunctional network interactions.
1. Introduction
Stroke causes adult disability in approximately 800,000 adults annually in the United States [1]. Unilateral upper motor weakness, known as hemiparesis, occurs in 77% of new stroke cases [2]. Hemiparesis frequently persists into the chronic stage of stroke; 65% of chronic stroke patients report reduced motor function 6 months after stroke [3,4]. Patients rarely obtain substantial motor improvement 3 months after a stroke, with residual motor deficits effectively becoming permanent [5–11]. Behavioral adaptations instead of spontaneous recovery generally underlie subsequent improvements [9]. Recent innovations in rehabilitation techniques, however, offer new opportunities for motor recovery, even in the chronic stage.
The efficacy of brain–computer interfaces (BCIs) for post-stroke motor rehabilitation has been demonstrated with a variety of designs [12]. However, there is a lack of consensus regarding the neurophysiological mechanisms driving recovery through BCI [13–16], which necessitated further study. Functional recovery was previously shown in a severely impaired chronic stroke population treated with a BCI system using signals from the contralesional motor cortex [17]. The former study used cortical EEG signals to control a robotic hand orthosis. Additionally, the efficacy of BCI on motor recovery was linked to changes in EEG activity in motor regions within frequencies used for BCI [17]. Given that this contralesional BCI system, known as the IpsiHand (Neurolutions, Santa Cruz CA), recently received FDA market authorization and will be applied to stroke populations, understanding the mechanism of its clinical benefit is of high importance. Power fluctuations in alpha (8–12 Hz) and beta (13–25 Hz) frequencies are observed in motor cortex during motor activity [18,19]. These frequencies are also used for BCI control [17]. We therefore hypothesized BCI may have affected neural circuitry to facilitate motor recovery via experience-dependent plasticity. However, previously recorded EEG signals only assess broad cortical regions with limited anatomic specificity. Here, we used functional MR imaging to study whether BCI therapy affected functional connectivity organization in the motor cortex and cerebellum.
Networks of correlated spontaneous brain activity during rest have been extensively described using functional MRI (fMRI) [20–22]. Strokes disrupt ‘functional connectivity’ networks [23–26]. Furthermore, the extent of network disruption correlated with stroke-induced impairments in multiple behavioral domains [23,25–27]. Strokes altered network modularity, typically by a decrease and then a partial recovery in association with behavioral improvements [25,28,29]. Connectivity changes between specific regions have also been implicated in stroke recovery [30–32]. Further, performance on motor function assessment tasks after a stroke was reduced with disrupted interhemispheric motor network connectivity [24,33]. Thus, recovery from stroke induced by BCI might involve changes in resting-state functional connectivity (rsFC).
The objective of the current study was to determine whether an EEG-driven BCI controlled by motor signals from the unaffected hemisphere reorganized brain networks for motor control. Based on previous reports linking motor network organization with post-stroke motor function, we hypothesized that motor recovery achieved during BCI therapy would change motor network connectivity, and that these rsFC changes in motor systems would correlate with the strength of recovery. Increases in interhemispheric connectivity, and decreases in intrahemispheric connectivity have previously been reported during stroke recovery [24,25,30,33–35]. Consequently, we hypothesized motor recovery via BCI would lead to similar patterns of change in inter- and intrahemispheric rsFC. The unexpected findings in this study suggest a potential novel recovery mechanism associated with BCI induced recovery in chronic stroke.[…]
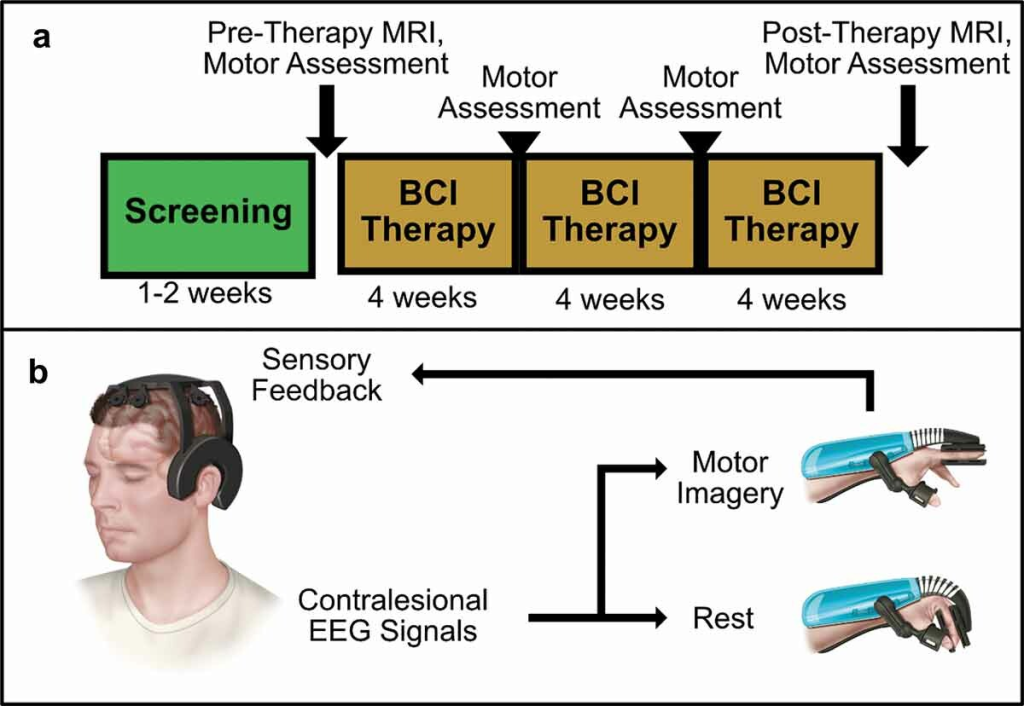
Figure 1. BCI Intervention protocol and system design overview. (a) Protocol Timeline. Screening for EEG feature frequency and inclusion and exclusion criteria occur over several sessions in a 1–2 week period. Following screening, patients undergo an MRI scan and motor assessments before receiving their BCI device. Patients perform BCI therapy for 12 weeks at home, returning every 4 weeks for motor assessments. A final MRI scan and motor assessment is performed after 12 weeks of therapy. (b) BCI System Design.
