Posts Tagged Motor network
[ARTICLE] Motor Network Reorganization Induced in Chronic Stroke Patients with the Use of a Contralesionally-Controlled Brain Computer Interface – Full Text
Posted by Kostas Pantremenos in REHABILITATION on July 6, 2022
ABSTRACT
Upper extremity weakness in chronic stroke remains a problem not fully addressed by current therapies. Brain–computer interfaces (BCIs) engaging the unaffected hemisphere are a promising therapy that are entering clinical application, but the mechanism underlying recovery is not well understood. We used resting state functional MRI to assess the impact a contralesionally driven EEG BCI therapy had on motor system functional organization. Patients used a therapeutic BCI for 12 weeks at home. We acquired resting-state fMRI scans and motor function data before and after the therapy period. Changes in functional connectivity (FC) strength between motor network regions of interest (ROIs) and the topographic extent of FC to specific ROIs were analyzed. Most patients achieved clinically significant improvement. Motor FC strength and topographic extent decreased following BCI therapy. Motor recovery correlated with reductions in motor FC strength across the entire motor network. These findings suggest BCI-mediated interventions may reverse pathologic strengthening of dysfunctional network interactions.
1. Introduction
Stroke causes adult disability in approximately 800,000 adults annually in the United States [1]. Unilateral upper motor weakness, known as hemiparesis, occurs in 77% of new stroke cases [2]. Hemiparesis frequently persists into the chronic stage of stroke; 65% of chronic stroke patients report reduced motor function 6 months after stroke [3,4]. Patients rarely obtain substantial motor improvement 3 months after a stroke, with residual motor deficits effectively becoming permanent [5–11]. Behavioral adaptations instead of spontaneous recovery generally underlie subsequent improvements [9]. Recent innovations in rehabilitation techniques, however, offer new opportunities for motor recovery, even in the chronic stage.
The efficacy of brain–computer interfaces (BCIs) for post-stroke motor rehabilitation has been demonstrated with a variety of designs [12]. However, there is a lack of consensus regarding the neurophysiological mechanisms driving recovery through BCI [13–16], which necessitated further study. Functional recovery was previously shown in a severely impaired chronic stroke population treated with a BCI system using signals from the contralesional motor cortex [17]. The former study used cortical EEG signals to control a robotic hand orthosis. Additionally, the efficacy of BCI on motor recovery was linked to changes in EEG activity in motor regions within frequencies used for BCI [17]. Given that this contralesional BCI system, known as the IpsiHand (Neurolutions, Santa Cruz CA), recently received FDA market authorization and will be applied to stroke populations, understanding the mechanism of its clinical benefit is of high importance. Power fluctuations in alpha (8–12 Hz) and beta (13–25 Hz) frequencies are observed in motor cortex during motor activity [18,19]. These frequencies are also used for BCI control [17]. We therefore hypothesized BCI may have affected neural circuitry to facilitate motor recovery via experience-dependent plasticity. However, previously recorded EEG signals only assess broad cortical regions with limited anatomic specificity. Here, we used functional MR imaging to study whether BCI therapy affected functional connectivity organization in the motor cortex and cerebellum.
Networks of correlated spontaneous brain activity during rest have been extensively described using functional MRI (fMRI) [20–22]. Strokes disrupt ‘functional connectivity’ networks [23–26]. Furthermore, the extent of network disruption correlated with stroke-induced impairments in multiple behavioral domains [23,25–27]. Strokes altered network modularity, typically by a decrease and then a partial recovery in association with behavioral improvements [25,28,29]. Connectivity changes between specific regions have also been implicated in stroke recovery [30–32]. Further, performance on motor function assessment tasks after a stroke was reduced with disrupted interhemispheric motor network connectivity [24,33]. Thus, recovery from stroke induced by BCI might involve changes in resting-state functional connectivity (rsFC).
The objective of the current study was to determine whether an EEG-driven BCI controlled by motor signals from the unaffected hemisphere reorganized brain networks for motor control. Based on previous reports linking motor network organization with post-stroke motor function, we hypothesized that motor recovery achieved during BCI therapy would change motor network connectivity, and that these rsFC changes in motor systems would correlate with the strength of recovery. Increases in interhemispheric connectivity, and decreases in intrahemispheric connectivity have previously been reported during stroke recovery [24,25,30,33–35]. Consequently, we hypothesized motor recovery via BCI would lead to similar patterns of change in inter- and intrahemispheric rsFC. The unexpected findings in this study suggest a potential novel recovery mechanism associated with BCI induced recovery in chronic stroke.[…]
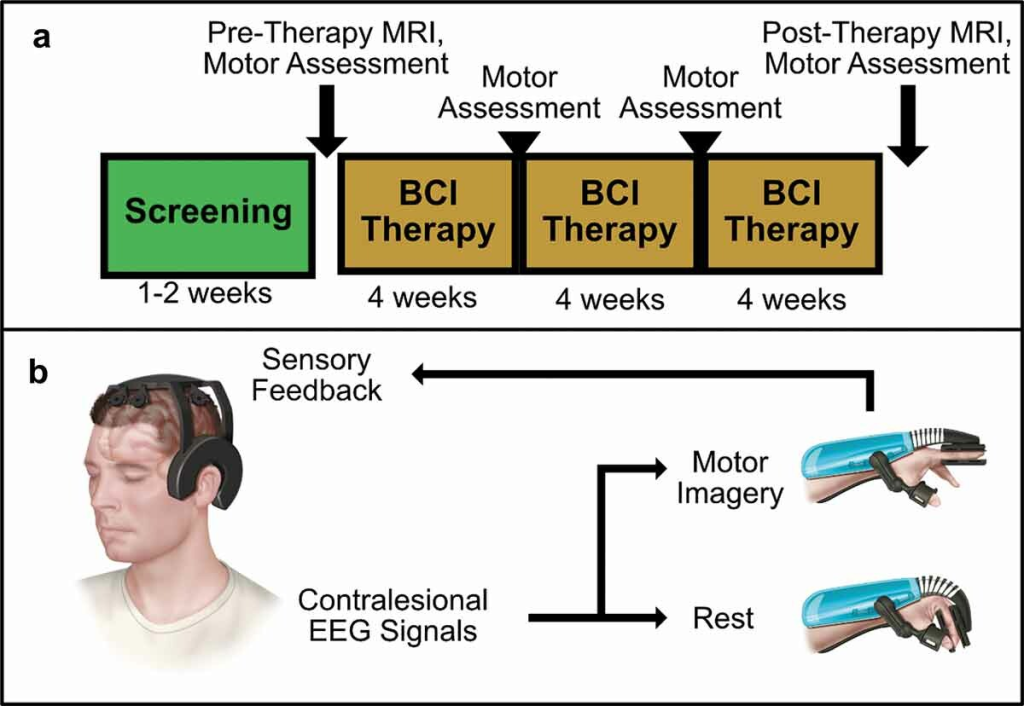
Figure 1. BCI Intervention protocol and system design overview. (a) Protocol Timeline. Screening for EEG feature frequency and inclusion and exclusion criteria occur over several sessions in a 1–2 week period. Following screening, patients undergo an MRI scan and motor assessments before receiving their BCI device. Patients perform BCI therapy for 12 weeks at home, returning every 4 weeks for motor assessments. A final MRI scan and motor assessment is performed after 12 weeks of therapy. (b) BCI System Design.
[Dissertation] BRAIN CONNECTIVITY CHANGES AFTER STROKE AND REHABILITATION – Full Text PDF
Posted by Kostas Pantremenos in Uncategorized on July 28, 2015
ABSTRACT
Several cortical and subcortical areas of brain interact coherently during various tasks such as motor-imagery (MI) and motor-execution (ME) and even during resting-state (RS). How these interactions are affected following stroke and how the functional organization is regained from rehabilitative treatments as people begin to recover have not been systematically studied. Role of primary motor area during MI task and how this differs during ME task are still questions of interest.
To answer such questions, we recorded functional magnetic resonance imaging (fMRI) signals from 30 participants: 17 young healthy controls and 13 aged stroke survivors following stroke and following rehabilitation – either mental practice (MP) or combined session of mental practice and physical therapy (MP + PT). All the participants performed RS task whereas stroke survivors performed MI and ME tasks as well. We investigated the activity of motor network consisting of the left primary motor area (LM1), the right primary motor area (RM1), the left pre-motor cortex (LPMC), the right pre-motor cortex (RPMC) and the midline supplementary motor area (SMA).
In this dissertation, first, we report that during RS the causal information flow (i) between the regions was reduced significantly following stroke (ii) did not increase significantly after MP alone and (iii) among the regions after MP+PT increased significantly towards the causal flow values for young able-bodied people. Second, we found that there was suppressive influence of SMA on M1 during MI task where as the influence was unrestricted during ME task. We reported that following intervention the connection between PMC and M1 was stronger during MI task whereas along with connection from PMC to M1, SMA to M1 also dominated during ME task. Behavioral results showed significant improvement in sensation and motor scores and significant correlation between differences in Fugl-Meyer Assessment (FMA) scores and differences in causal flow values as well differences in endogenous connectivity measures before and after intervention.
We conclude that the spectra of causal information flow can be used as a reliable biomarker for evaluating rehabilitation in stroke survivors. These studies deepen our understanding of motor network activity during the recovery of motor behaviors in stroke. Understanding the stroke specific effective connectivity may be clinically beneficial in identifying effective treatments to maximize functional recovery in stroke survivors.
[Dissertation] BRAIN CONNECTIVITY CHANGES AFTER STROKE AND REHABILITATION – Full Text PDF
Posted by Kostas Pantremenos in Uncategorized on July 14, 2015
ABSTRACT
Several cortical and subcortical areas of brain interact coherently during various tasks such as motor-imagery (MI) and motor-execution (ME) and even during resting-state (RS). How these interactions are affected following stroke and how the functional organization is regained from rehabilitative treatments as people begin to recover have not been systematically studied. Role of primary motor area during MI task and how this differs during ME task are still questions of interest.
To answer such questions, we recorded functional magnetic resonance imaging (fMRI) signals from 30 participants: 17 young healthy controls and 13 aged stroke survivors following stroke and following rehabilitation – either mental practice (MP) or combined session of mental practice and physical therapy (MP + PT). All the participants performed RS task whereas stroke survivors performed MI and ME tasks as well. We investigated the activity of motor network consisting of the left primary motor area (LM1), the right primary motor area (RM1), the left pre-motor cortex (LPMC), the right pre-motor cortex (RPMC) and the midline supplementary motor area (SMA).
In this dissertation, first, we report that during RS the causal information flow (i) between the regions was reduced significantly following stroke (ii) did not increase significantly after MP alone and (iii) among the regions after MP+PT increased significantly towards the causal flow values for young able-bodied people. Second, we found that there was suppressive influence of SMA on M1 during MI task where as the influence was unrestricted during ME task. We reported that following intervention the connection between PMC and M1 was stronger during MI task whereas along with connection from PMC to M1, SMA to M1 also dominated during ME task.
Behavioral results showed significant improvement in sensation and motor scores and significant correlation between differences in Fugl-Meyer Assessment (FMA) scores and differences in causal flow values as well differences in endogenous connectivity measures before and after intervention.
We conclude that the spectra of causal information flow can be used as a reliable biomarker for evaluating rehabilitation in stroke survivors. These studies deepen our understanding of motor network activity during the recovery of motor behaviors in stroke. Understanding the stroke specific effective connectivity may be clinically beneficial in identifying effective treatments to maximize functional recovery in stroke survivors.
