Posts Tagged Accelerometers
[Abstract] The use of wearable sensors to assess and treat the upper extremity after stroke: a scoping review
Posted by Kostas Pantremenos in Paretic Hand on August 5, 2021
Abstract
Purpose
To address the gap in the literature and clarify the expanding role of wearable sensor data in stroke rehabilitation, we summarized the methods for upper extremity (UE) sensor-based assessment and sensor-based treatment.
Materials and methods
The guideline outlined by the preferred reporting items for systematic reviews and meta-analysis extension for scoping reviews was used to complete this scoping review. Information pertaining to participant demographics, sensory information, data collection, data processing, data analysis, and study results were extracted from the studies for analysis and synthesis.
Results
We included 43 articles in the final review. We organized the results into assessment and treatment categories. The included articles used wearable sensors to identify UE functional motion, categorize motor impairment/activity limitation, and quantify real-world use. Wearable sensors were also used to augment UE training by triggering sensory cues or providing instructional feedback about the affected UE.
Conclusions
Sensors have the potential to greatly expand assessment and treatment beyond traditional clinic-based approaches. This capability could support the quantification of rehabilitation dose, the nuanced assessment of impairment and activity limitation, the characterization of daily UE use patterns in real-world settings, and augment UE training adherence for home-based rehabilitation.
- IMPLICATIONS FOR REHABILITATION
- Sensor data have been used to assess UE functional motion, motor impairment/activity limitation, and real-world use.
- Sensor-assisted treatment approaches are emerging, and may be a promising tool to augment UE adherence in home-based rehabilitation.
- Wearable sensors may extend our ability to objectively assess UE motion beyond supervised clinical settings, and into home and community settings.
[Abstract] A Smart-Band Operated Wrist Rehabilitation Robot
Posted by Kostas Pantremenos in Paretic Hand, Rehabilitation robotics on December 12, 2020
Abstract
Many people in the world are increasingly suffering from stroke issues. Survivors often tend to suffer from hemiplegia or related conditions, in which some portion of their body may be rendered useless. The wrist is one such part. But this injury can be recovered by conventional rehabilitation processes like physical therapy. In this paper, a device for robot-assisted physical therapy is presented for wrist rehabilitation. It can overcome the lack of availability of physical therapists and reduce the cost incurred in long-term therapy. Also, it can provide accurate regular exercises without missing any step even in the absence of the therapist. These two DOF robotic devices can learn the physical exercise (i.e. wrist-based movements) from the trained therapist through an electronic smart-band. It can also replicate these exercises when the patient wears this device over his/her wrist. Here, an accelerometer sensor and a magnetometer sensor-based smart-band are used for recognizing the wrist motions like flexion, extension, abduction, and adduction. The objective of this preliminary work is to drive accurately all the motor actuators which are attached to the robot and calibrate the feedback sensor to reflect the movement of the smart-band. In the future, this robot can be used as a teleoperated rehabilitation device through an IoT platform.
[ARTICLE] Multi-Sensor Validation Approach of an End-Effector-Based Robot for the Rehabilitation of the Upper and Lower Limb – Full Text
Posted by Kostas Pantremenos in Paretic Hand, Rehabilitation robotics on October 27, 2020
Abstract
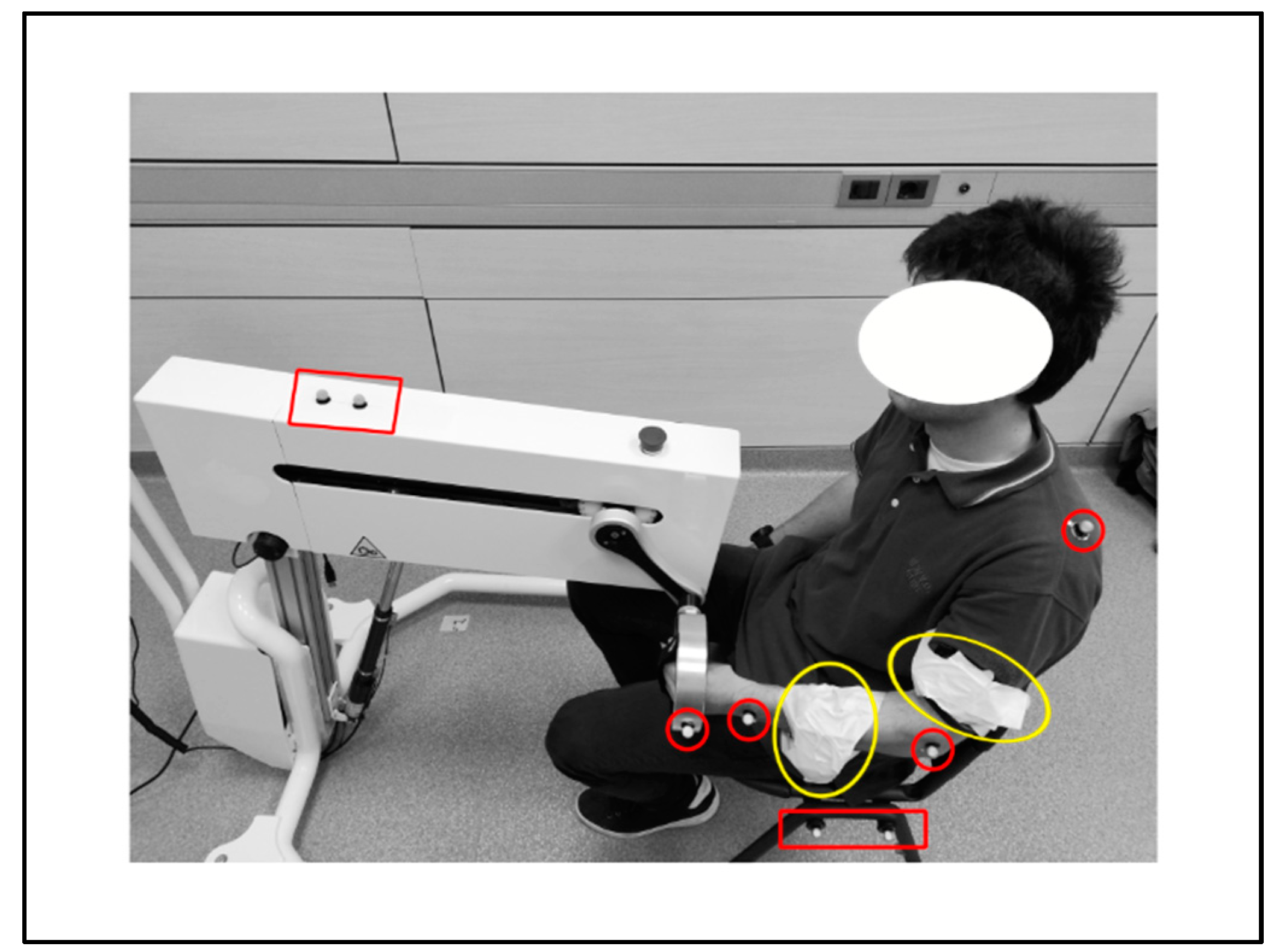
End-effector-based robots are widely adopted by physiotherapists and caregivers as support in the delivery of the rehabilitation training to the patient. The validation of these devices presents critical aspects, since the system performance must be assessed analyzing the movement performed by the subject limb, i.e., elements outside the device. This paper presents a multi-sensor approach for the validation of an innovative end-effector-based device, comparing different measurement strategies for evaluating the system effectiveness in imposing an expected training. The study was performed monitoring the movement induced by the device on the upper limb of a young male healthy subject during a set of fictitious rehabilitation sessions. The kinematic structure of the device is characterized by a compact differential mechanism with two degrees of freedom. A sequence of repetitions of a planar reaching pattern was analyzed as illustrative training task. A kinematic model of subject and system was developed, and the kinematics of a set of specific landmark points on the subject limb was evaluated. Data obtained from two measurement systems were compared: (1) an optoelectronic system with two cameras and eight skin passive markers, and (2) two triaxial accelerometers. Results were analyzed in MATLAB and R environment, revealing a high repeatability of the limb movement. Although both the measurement systems allow evaluating the acceleration of subject’s arm and forearm, accelerometers should be preferred for punctual analysis, like components optimizations, whereas optical markers provide a general overview of the system, particularly suitable for the functional design process.
1. Introduction
The motor skills reduction in subjects affected by neurologically based disorders, like stroke, spinal cord injuries and traumatic brain lesions, strongly influences the quality of life [1,2,3]. In particular, the ability of independently and self-sufficiently execute everyday motor tasks is greatly reduced by extremities functional limitations [4]. For this reason, more than restoring the capacity of realizing a task in the natural way, the primary aim of rehabilitation techniques is allowing the execution of the lost motor functions, re-educating the subjects to coordinate movements. Besides, motor and neuro-motor rehabilitation, combined with the use of orthoses and functional electrical stimulation, improves also the subject mental abilities and prevents secondary complications such as spasticity, muscle atrophy and osteoporosis [5].The physical rehabilitation process begins with a preliminary analysis of the patient’s residual abilities, suitable for identifying the most effective rehabilitation protocol. According to literature, biofeedback and robot-assisted therapy, as well as virtual reality training, intensify the rehabilitation therapy allowing the accurate repetition of motor patterns [6]. Indeed, onset, intensity, duration and task-orientation of the training significantly affect the achievement of positive outcomes. As literature enlightens, repetitive training generates functional improvements which depend on patients inclusion criteria and time elapsed from the stroke, but also on the repetitions quantity [7]. In fact, scientific evidences suggest to extend the duration of training sessions, since longer sessions have better effects on motor functions [7,8].In recent literature, many works deal with the use of robotic devices for rehabilitation purposes, offering a wide variety of solutions for the upper and lower limbs rehabilitation, both in clinical environments and at home [5,9,10,11]. Several devices may provide a different kind of motion assistance, like passive or active mobilization, as well as haptic assistance or coaching [9]. Active devices, presenting at least one actuator, can induce the movement of specific parts of the limb, performing active or passive exercises. Moreover, the device may support the subject, which actively performs the task by moving the limb; on the contrary, in passive exercises the patient movement is guided by the device during the rehabilitation session.An alternative taxonomy classifies devices considering the mechanical design. Actually they can be (1) end-effector-based, i.e., the contact between machine and patient’s limb arises only at the end-effector level (e.g., MIT Manus [12]), or (2) exoskeleton-based, in which the mechanical configuration of the device mirrors the limb’s skeletal structure [13,14,15]; in those devices the contact between subject and system is distributed along the limb with multiple contact areas. Literature also presents many devices that combine these two structures, like the MIME-RiceWrist rehabilitation system [16].Besides, some devices are characterized by specific and significant features. Among them, reconfigurability represents the capability of the system of changing its mechanical structure, adapting it to different use conditions, or the ability to follow the subject necessities (e.g., MUNDUS [6]). Back-drivability describes instead the possibility of the patient to induce the movement of the system when the device is in passive state (e.g., HWARD [17]). Mechanical structure of the system, as well as type, number and location of the actuators determine the allowed movements of the device and the degrees of freedom (DOFs) of the system consequently. As the literature reports, most of the devices allow three-dimensional movements [18], whereas only a restricted group of devices enable the movement on a specific plane (e.g., ARC-MIME [19]).Focusing on the control of the devices, several signals may be evaluated, like externally imposed triggers [20], kinematic or dynamic signals from the device [21,22], as well as biomechanical signals from the subject, such as data derived from surface electromyography (sEMG [23,24]). Nonetheless, biomechanical systems can be considered characterized by low dynamics phenomena, and rehabilitation training even more, given the low velocities required for a correct training [25].Literature provides numerous examples of multi-sensor validation in clinical or rehabilitative contexts when considering the human motion [26,27], whereas multiple units of the same sensor are generally used when validating devices [28].Within this complex context, the validation process of new rehabilitation devices becomes critical, since the true analysis dimension for evaluating the system performance coincides with the analysis of the motion performed by the subject, referring therefore to elements outside the device. Hence, the system to monitor can include the device, but mainly focuses on the final user, i.e., the patient. We can describe some validation methods as device-oriented, meaning that the validation is pursued through the comparison of the performance, as recorded by the rehabilitation system, with those detected by the sensors located along the device. This is the typical condition for exoskeleton devices, in which the design of the system, with the distributed contact between machine and subject, justifies the hypothesis of negligible approximation errors between motion profile realized by device and movement of the patient limb. In this case, sensors like the inertial measurement unit (IMU) [29] or camera-based systems [30] are mostly used. Besides, according to the same rationale, other validation methods can be defined as user-oriented, since the analysis is performed detecting the patient movement, thanks to wearable sensors like EMG sensors [31] and optoelectronic systems [29] placed on significant landmarks of the testing subjects. This approach is necessary for end-effector based devices, since the process to monitor is partially independent from the device constraints, and differently from the device-oriented methods, it demands for the identification of a proper kinematic or dynamic model of the subject.In this work a multi-sensor validation approach is investigated and an innovative rehabilitation device was considered for the study. The device is an end-effector-based robotic system that has been developed within the SIMeRiON (Innovative Mechatronics System for Orthopedic and Neurological Rehabilitation) project, funded by Regione Lombardia [32]. The device is back-drivable and reconfigurable, presents an electro-mechanical actuation system, and is able to provide passive, active and assisted rehabilitation [32,33]. The mechanical system is based on a compact differential system and is characterized by two DOFs; this allows implementing every kind of motion profile within a plane. The device performance is analyzed evaluating the movement induced by the device on the upper limb of a healthy subject in a sequence of repetitions. The movement characteristics are investigated monitoring specific landmark points of the subject’s limb, with the aim of verifying the system effectiveness in imposing the expected training. The kinematics of those points has been detected thanks to (1) an optical marker-based tracking system, and (2) an inertial sensor-based system. Acquired data have been compared to evaluate strength points and drawbacks of each measuring strategy for the proper tuning of the system model; in fact, the kinematic model of subject and system has been defined and used as reference for the interpretation of the collected data. In the next section, a synthetic description of the device is reported, and the adopted methods for the performed data treatment are described. Results are then presented and discussed in the following sections, whereas main strength points and limits of the work are finally described in the conclusions.[…]
[Abstract] Predicting and Monitoring Upper-Limb Rehabilitation Outcomes Using Clinical and Wearable Sensor Data in Brain Injury Survivors
Posted by Kostas Pantremenos in Paretic Hand, TBI on October 5, 2020
Abstract
Objective: Rehabilitation specialists have shown considerable interest for the development of models, based on clinical data, to predict the response to rehabilitation interventions in stroke and traumatic brain injury survivors. However, accurate predictions are difficult to obtain due to the variability in patients’ response to rehabilitation interventions. This study aimed to investigate the use of wearable technology in combination with clinical data to predict and monitor the recovery process and assess the responsiveness to treatment on an individual basis.
Methods: Gaussian Process Regression-based algorithms were developed to estimate rehabilitation outcomes (i.e., Functional Ability Scale scores) using either clinical or wearable sensor data or a combination of the two.
Results: The algorithm based on clinical data predicted rehabilitation outcomes with a Pearson’s correlation of 0.79 compared to actual clinical scores provided by clinicians but failed to model the variability in responsiveness to the intervention observed across individuals. In contrast, the algorithm based on wearable sensor data generated rehabilitation outcome estimated with a Pearson’s correlation of 0.91 and modeled the individual responses to rehabilitation more accurately. Furthermore, we developed a novel approach to combine estimates derived from the clinical data and the sensor data using a constrained linear model. This approach resulted in a Pearson’s correlation of 0.94 between estimated and clinician-provided scores.
Conclusion: This algorithm could enable the design of patient-specific interventions based on predictions of rehabilitation outcomes relying on clinical and wearable sensor data.
Significance: This is important in the context of developing precision rehabilitation interventions.
[ARTICLE] Changes in actual arm-hand use in stroke patients during and after clinical rehabilitation involving a well-defined arm-hand rehabilitation program: A prospective cohort study – Full Text
Posted by Kostas Pantremenos in Paretic Hand, REHABILITATION on April 18, 2019
Abstract
Introduction
Improvement of arm-hand function and arm-hand skill performance in stroke patients is reported by many authors. However, therapy content often is poorly described, data on actual arm-hand use are scarce, and, as follow-up time often is very short, little information on patients’ mid- and long-term progression is available. Also, outcome data mainly stem from either a general patient group, unstratified for the severity of arm-hand impairment, or a very specific patient group.
Objectives
To investigate to what extent the rate of improvement or deterioration of actual arm-hand use differs between stroke patients with either a severely, moderately or mildly affected arm-hand, during and after rehabilitation involving a well-defined rehabilitation program.
Methods
Design: single–armed prospective cohort study. Outcome measure: affected arm-hand use during daily tasks (accelerometry), expressed as ‘Intensity-of arm-hand-use’ and ‘Duration-of-arm-hand-use’ during waking hours. Measurement dates: at admission, clinical discharge and 3, 6, 9, and 12 months post-discharge. Statistics: Two-way repeated measures ANOVAs.
Results
Seventy-six patients (63 males); mean age: 57.6 years (sd:10.6); post-stroke time: 29.8 days (sd:20.1) participated. Between baseline and 1-year follow-up, Intensity-of-arm-hand-use on the affected side increased by 51%, 114% and 14% (p < .000) in the mildly, moderately and severely affected patients, respectively. Similarly, Duration-of-arm-hand-use increased by 26%, 220% and 161% (p < .000). Regarding bimanual arm-hand use: Intensity-of-arm-hand-use increased by 44%, 74% and 30% (p < .000), whereas Duration-of-arm-hand-use increased by 10%, 22% and 16% (p < .000).
Conclusion
Stroke survivors with a severely, moderately or mildly affected arm-hand showed different, though (clinically) important, improvements in actual arm-hand use during the rehabilitation phase. Intensity-of-arm-hand-use and Duration-of-arm-hand-use significantly improved in both unimanual and bimanual tasks/skills. These improvements were maintained until at least 1 year post-discharge.
Introduction
After stroke, the majority of stroke survivors experiences significant arm-hand impairments [1, 2] and a decreased use of the paretic arm and hand in daily life [3]. The actual use of the affected hand in daily life performance depends on the severity of the arm-hand impairment [4–6] and is associated with perceived limitations in participation [7, 8]. Severity of arm-hand impairment is also associated with a decrease of health-related quality of life [9], restricted social participation [10], and subjective well-being [11, 12].
Numerous interventions and arm-hand rehabilitation programs have been developed in order to resolve arm-hand impairments in stroke patients [6, 13]. In the Netherlands, a number of stroke units in rehabilitation centres implemented a well-described ‘therapy-as-usual’ arm-hand rehabilitation program, called CARAS (acronym for: Concise Arm and hand Rehabilitation Approach in Stroke)[14], serving a broad spectrum of stroke patients across the full stroke severity range of arm-hand impairments. The arm-hand rehabilitation program has been developed to guide clinicians in systematically designing arm-hand rehabilitation, tailored towards the individual patient’s characteristics while keeping control over the overall heterogeneity of this population typically seen in stroke rehabilitation centres. A vast majority of stroke patients who participated in CARAS improved on arm-hand function (AHF), on arm-hand skilled performance (AHSP) capacity and on (self-) perceived performance, both during and after clinical rehabilitation [15]. The term ‘arm-hand function’ (AHF) refers to the International Classification of Functioning (ICF) [16] ‘body function and structures level’. The term ‘arm-hand skilled performance’ (AHSP) refers to the ICF ‘activity level’, covering capacity as well as both perceived performance and actual arm-hand use [17].
Improved AHF and/or AHSP capacity do not automatically lead to an increase in actual arm-hand use and do not guarantee an increase of performing functional activities in daily life [18–20]. Improvements at function level, i.e. regaining selectivity, (grip) strength and/or grip performance, do not automatically lead to improvements experienced in real life task performance of persons in the post-stroke phase who live at home [18, 21]. Next to outcome measures regarding AHF, AHSP capacity and (self-) perceived AHSP, which are typically measured in controlled conditions, objective assessment of functional activity and actual arm-hand use outside the testing situation is warranted [22, 23].
Accelerometry can be used to reliably and objectively assess actual arm-hand use during daily task performance [24–32]and has been used in several studies to detect arm-hand movements and evaluate arm-hand use in the post-stroke phase [20, 33–35]. Previous studies have demonstrated that, in stroke patients, movement counts, as measured with accelerometers, are associated with the use of the affected arm-hand (Motor Activity Log score) [36, 37] and, at function level, with the Fugl-Meyer Assessment [38]. Next to quantifying paretic arm-hand use, accelerometers have also been used to provide feedback to further enhance the use of the affected hand in home-based situations [39]. Most studies consist of relatively small [27, 30, 40–44] and highly selected study populations [45] with short time intervals between baseline and follow-up measurements. As to our knowledge, only a few studies monitored arm-hand use in stroke patients for a longer period, i.e. between time of discharge to a home situation or till 6 to 12 months after stroke [19, 44, 46]. However, they used a relatively small study sample and their intervention aimed at arm-hand rehabilitation was undefined. Both studies of Connell et al. and Uswatte et al. describe a well-defined arm hand intervention where accelerometry data were used as an outcome measure [27, 47]. However, the study population described by Connell et al. consisted of a relative small and a relative mildly impaired group of chronic stroke survivors. The study population described by Uswatte et al. consisted of a large group of sub-acute stroke patients within strict inclusion criteria ranges [37], who, due to significant spontaneous neurologic recovery within this sub-acute phase, had a mildly impaired arm and hand [48, 49]. This means that the group lacked persons with a moderately to severely affected arm-hand, who are commonly treated in the daily rehabilitation setting.
The course of AHF and AHSP of a broad range of sub-acute stroke patients during and after rehabilitation involving a well-defined arm-hand rehabilitation program (i.e. CARAS) [14] has been reported by Franck et al. [15]. The present paper provides data concerning actual arm-hand use in the same study population, and focuses on two objectives. The first aim is to investigate changes in actual arm-hand use across time, i.e. during and after clinical rehabilitation, within a stroke patient group typically seen in daily medical rehabilitation practice, i.e. covering a broad spectrum of arm-hand problem severity levels, who followed a well-described arm-hand treatment regime. The second aim is to investigate to what extent improvement (or deterioration) regarding the use of the affected arm-hand in daily life situations differs between patient categories, i.e. patients with either a severely, moderately or mildly impaired arm-hand, during and after their rehabilitation, involving a well-defined arm-hand rehabilitation program.[…]
Fig 3. Mean values for Intensity-of-arm-hand-use during uptime for subgroups 1, 2 and 3.
T = time; bl = baseline; cd = clinical discharge; m = month; Solid line = subgroup 1; Dotted line = subgroup 2; Dashed line = subgroup 3.
https://doi.org/10.1371/journal.pone.0214651.g003
[ARTICLE] Predicting Daily Use of the Affected Upper Extremity 1 Year after Stroke
Posted by Kostas Pantremenos in Paretic Hand on December 23, 2014
Background
The ultimate goal of upper extremity (UE) stroke rehabilitation is for the individual with stroke to return using their arms and hands during daily activities in their own environment. No studies have monitored arm use as individuals with stroke transition from rehabilitation to the home setting. This longitudinal study compared the functional ability and daily use of the affected UE of individuals with stroke between discharge to home and 12 months after stroke and predicted the UE daily use 12 months after stroke.
Methods
Participants were assessed on discharge to home from rehabilitation and at 12 months after stroke. UE daily use was measured by wrist accelerometers and self-report by the Motor Activity Log (MAL). Multivariate logistic regression models were used to predict UE daily use 12 months after stroke.
Results
The UE functional ability improved significantly from discharge to 12 months after stroke. The amount of self-report UE daily use significantly improved (z = −2.9, P = .004), but accelerometer activity counts did not (z = −0.15, P = .88), and the daily use of the nonaffected UE was 3 times more than the affected UE. After controlling for age and accelerometer daily use on discharge, UE variables of movement, function, dexterity, and strength accounted for an additional 10.9%-13.6% of the variance for accelerometer readings. After controlling for gender and MAL daily use on discharge, UE variables accounted for an additional 7%-12% of the variance for the MAL.
Conclusions
UE daily use 12 months after stroke is very limited despite the motor and functional improvement. Enhanced motor and functional ability at discharge predicts more UE daily use at 12 months after stroke. Interventions that monitor and encourage these individuals to use their UE are required to ensure that functional gains translate to daily use.
via Predicting Daily Use of the Affected Upper Extremity 1 Year after Stroke.
