Posts Tagged real-time systems
[Abstract + References] Real-Time Evaluation of Hand Motor Function Recovery in Home Use Finger Rehabilitation Device Using Gaussian Process Regression – IEEE Conference Publication
Posted by Kostas Pantremenos in Paretic Hand, Rehabilitation robotics, Tele/Home Rehabilitation on December 23, 2020
Abstract:Continuous hand rehabilitation after discharge is important for hemiplegic patients to regain an independent finger movement. However, most patients cannot rehabilitate by themselves without therapists. For this problem, robotic rehabilitation has been investigated to support patients even at home. Most of the programs performed by these robots are focusing on the assistance for voluntary movement. However, the approach to the voluntary movement is not enough for regaining dexterous movement. Voluntary suppression of body parts that should not move is important. However, previous studies focusing on voluntary suppression are few. In this paper, we show a detailed program for voluntary suppression rehabilitation. The program is performed by our robotic finger rehabilitation device aiming at home use. In this program, a patient is requested to flex and extend an index finger independently. During moving, individual pressure sensors monitor the other fingers. If the device detects unnecessary movements such as compensatory movement at some fingers, the patient is notified that unnecessary movements are found there. The detection is based on 3σ range of healthy subject’s finger pressure data which was constructed by using Gaussian Process Regression. Through experiments with hemiplegic patients, we have shown that the frequency of deviation of patients’ data from 3σ range of healthy subjects decreases according to the degree of recovery.
References
1.C. L. Jones, F. Wang, R. Morrison, N. Sarkar and D. G. Kamper, “Design and Development of the Cable Actuated Finger Exoskeleton for Hand Rehabilitation Following Stroke”, IEEE/ASME Transactions on Mechatronics, vol. 19, no. 1, pp. 131-140, 2014.Show Context View Article Full Text: PDF (740KB) Google Scholar 2.D. Leonardis et al., “An EMG-Controlled Robotic Hand Exoskeleon for Bilateral Rehabilitation”, IEEE Transactions on Haptics, vol. 8, no. 2, pp. 140-151, 2015.Show Context View Article Full Text: PDF (1941KB) Google Scholar 3.S. Biggar and W. Yao, “Design and Evaluation of a Soft and Wearable Robotic Glove for Hand Rehabilitation”, IEEE Transactions on Neural Systems and Rehabilitation Engineering, vol. 24, no. 10, pp. 1071-1080, 2016.Show Context View Article Full Text: PDF (1597KB) Google Scholar 4.P. Polygerinos, K. C. Galloway, S. Sanan, M. Herman and C. J. Walsh, “EMG Controlled Soft Robotic Glove for Assistance during Activities of Daily Living”, 2015 IEEE International Conference on Rehabilitation Robotics (ICORR), pp. 55-60, 2015.Show Context View Article Full Text: PDF (2640KB) Google Scholar 5.I. Ben Abdallah, Y. Bouteraa and C. Rekik, “Design and Development of 3D Printed Myoelectric Robotic Exoskeleton for Hand Rehabilitation”, International Journal on Smart Sensing and Intelligent Systems, vol. 10, pp. 341-366, 2017.Show Context CrossRef Google Scholar 6.K. Yamamoto, Y. Furudate, K. Chiba, Y. Ishida and S. Mikami, “Home Robotic Device for Rehabilitation of Finger Movement of Hemiplegia Patients”, 2017 IEEE/SICE International Symposium on System Integration (SII), pp. 300-305, 2017.Show Context View Article Full Text: PDF (1305KB) Google Scholar 7.C. D. Takahashi, L. Der-Yeghiaian, V. Le, R. R. Motiwala and S. C. Cramer, “Robot-based Hand Motor Therapy after Stroke”, Brain, vol. 131, no. Pt 2, pp. 425-437, 2008.Show Context CrossRef Google Scholar 8.L. Dovat et al., A Technique to Train Finger Coordination and Independence after Stroke, Disability and Rehabilitation:Assistive Technology, vol. 5, no. 4, pp. 279-287, 2010.Show Context Google Scholar 9.Y. Furudate, N. Onuki, K. Chiba, Y. Ishida and S. Mikami, “Automated Evaluation of Hand Motor Function Recovery by Using Finger Pressure Sensing Device for Home Rehabilitation”, 2018 IEEE 18th International Conference on Bioinformatics and Bioengineering (BIBE), pp. 207-214, 2018.Show Context View Article Full Text: PDF (500KB) Google Scholar 10.Y. Furudate, N. Onuki, K. Chiba, Y. Ishida and S. Mikami, “Hand Motor Function Evaluation by Integrating Multi-Tasks Using Home Rehabilitation Device”, 2020 IEEE 2nd Global Conference on Life Sciences and Technologies (LifeTech), pp. 272-274, 2020.Show Context View Article Full Text: PDF (2090KB) Google Scholar
[Abstract] A Home-based Bilateral Rehabilitation System with sEMG-based Real-time Variable Stiffness
Posted by Kostas Pantremenos in Paretic Hand, REHABILITATION, Rehabilitation robotics, Tele/Home Rehabilitation on October 19, 2020
Abstract
Bilateral rehabilitation allows patients with hemiparesis to exploit the cooperative capabilities of both arms to promote the recovery process. Although various approaches have been proposed to facilitate synchronized robot-assisted bilateral movements, few studies have focused on addressing the varying joint stiffness resulting from dynamic motions. This paper presents a novel bilateral rehabilitation system that implements a surface electromyography (sEMG)-based stiffness control to achieve real-time stiffness adjustment based on the user’s dynamic motion. An sEMG-driven musculoskeletal model that incorporates muscle activation and muscular contraction dynamics is developed to provide reference signals for the robot’s real-time stiffness control. Preliminary experiments were conducted to evaluate the system performance in tracking accuracy and comfortability, which showed the proposed rehabilitation system with sEMG-based real-time stiffness variation achieved fast adaption to the patient’s dynamic movement as well as improving the comfort in robot-assisted bilateral training.
[ARTICLE] Adaptive Treadmill-Assisted Virtual Reality-Based Gait Rehabilitation for Post-Stroke Physical Reconditioning—a Feasibility Study in Low-Resource Settings – Full Text
Posted by Kostas Pantremenos in Gait Rehabilitation - Foot Drop, REHABILITATION, Virtual reality rehabilitation on May 26, 2020
Abstract

Physiological Cost Index sensitive Adaptive Response Technology (PCI-ART) for post-stroke physical reconditioning. Note: PCI- Physiological Cost Index; SST-Single Support Time; AL- Affected limb; UAL- Unaffected limb.
Introduction
Neurological disorders, such as stroke is a leading cause of disability with a prevalence rate of 424 in 100,000 individuals in India [1]. Often, these patients suffer from functional disabilities, heterogeneous physical deconditioning along with deteriorated cardiac functioning [2], [3] and a sedentary lifestyle immediately following stroke [4]. A deconditioned patient requires reconditioning of his/her cardiac capacity and ambulation capabilities that can be achieved through individualized rehabilitation [5]. This needs to be done under the supervision of a clinician who can monitor one’s functional capability, cardiac capacity and gait performance thereby recommending an appropriate dosage of the gait rehabilitation exercise intensity to the patient along with feedback. Such gait rehabilitation is crucial since about 80% of these patients have been reported to suffer from gait-related disorders [6] along with more energy expenditure than able-bodied individuals [7] often accompanied with reduced cardiac capacity [2], [4]. However, given the low doctor-to-patient ratio [8], lack of rehabilitation facilities and patients being released early from rehabilitation clinics followed by home-based exercise [9], particularly in developing countries like India, availing individualized rehabilitation services becomes difficult. Again, undergoing home-based exercises under clinician’s one-on-one supervision becomes difficult given the restricted healthcare resources, thereby limiting the rehabilitation outcomes [10]. Again, given the restricted healthcare resources, getting a clinician visiting the homes for delivering therapy sessions to patients is often costly causing the patients to miss the expert inputs on the exercise intensity suiting his/her exercise capability along with motivational feedback from the clinician [11]. This necessitates the use of a complementary technology-assisted rehabilitation platform that can be availed by the patient at his/her home [12] following a short stay at the rehabilitation clinic [13]. Again, it is preferred that this platform be capable of offering individualized gait exercise while varying the dosage of exercise intensity (based on the patient’s exercise capability) along with motivational feedback [14]. Additionally, exercise administered by this platform can be complemented with intermediate clinician-mediated assessments of rehabilitation outcomes, thereby reducing continuous demands on the restricted clinical resources. Thus, it is important to investigate the use of such technology-assisted gait exercise platforms that are capable of offering exercise based on one’s individualized capability along with motivational feedback.
Researchers have explored the use of technology-assisted solutions to offer rehabilitative gait exercises to these patients, along with presenting motivational feedback [15]–[16][17][18][19][20][21][22][23][24]. Specifically, investigators have used Virtual Reality (VR) coupled with a treadmill (having a limited footprint and making it suitable for home-based settings) while delivering individualized feedback [15] to the patient during exercise. Again, VR can help to project scenarios that can make the exercise engaging and interactive for a user [16]–[17][18][19]. In fact, Finley et al. have shown that the visual feedback offered by VR provides an optical flow that can induce changes in the gait performance (quantified in terms of gait parameters, e.g., Step Length, Step Symmetry, etc.) of such patients during treadmill-assisted walk [20]. Further, Jaffe et al. have reported positive implications of VR-based treadmill-assisted walking exercise on the gait performance of individuals with stroke [23], leading to improvement in their community ambulation [24]. These studies have shown the efficacy of the VR-based treadmill-assisted gait exercise platform to contribute towards gait rehabilitation of individuals suffering from stroke. Though promising, none of these platforms are sensitive to one’s individualized exercise capability and thus, in turn, could not decide an optimum dosage of exercise intensity suiting one’s capability, e.g., cardiac capacity and ambulation capability. This is particularly critical for individuals with stroke since they possess diminished exercise ability along with deteriorated cardiac functioning [2], [4].
From literature review, we find that after stroke, treadmill-assisted cardiac exercise programs can lead to one’s improved fitness and exercise capability [25]. For example, researchers have presented studies on Moderate-Intensity Continuous Exercise and High-Intensity Interval Training in which exercise protocols are individualized by a clinician based on one’s cardiac capacity while contributing to effective gait rehabilitation [26]–[27][28][29]. Though promising, these have not offered a progressive and adaptive exercise environment in which the dosage of exercise intensity is varied based on one’s cardiac capacity in real-time. Thus, the choice of optimum dosage of exercise intensity that can be individualized in real-time for a patient, still remains as inadequately explored [4]. For deciding the optimal dosage of rehabilitative exercise intensity, clinicians often refer to the guidelines recommended by the American College of Sports Medicine (ACSM) [30]. These guidelines suggest thresholds to decide the intensity of the exercise based on one’s metabolic energy consumption in terms of oxygen intake, heart rate, etc. Deciding the dosage of exercise intensity is crucial, particularly for individuals with stroke since their energy requirements have been reported to be 55-100% higher than that of their able-bodied counterparts [7]. Specifically, higher energy requirement often limits the capabilities of these patients and challenges their rehabilitation outcomes. This can be addressed if the technology-assisted gait exercise platform can offer individualized exercise (maintaining the safe exercise thresholds) based on the energy expenditure of the patients acquired in real-time during the exercise.
The energy expenditure can be defined as the cost of physical activity [4] and it is often expressed in terms of oxygen consumption or heart rate [31]. Thus, investigators have monitored the oxygen consumption and heart rate to estimate the energy expenditure of individuals with stroke during their walk [31], [32]. However, monitoring oxygen consumption during exercise requires a cumbersome setup [31], making it unsuitable for home-based rehabilitation. On the other hand, one’s heart rate (HR) can be monitored using portable solutions [33] that can be integrated with a treadmill in home-based settings. Researchers have explored treadmill-assisted gait exercise platforms that are sensitive to the user’s heart rate. For example, researchers have offered treadmill training to subjects with stroke in which some of them varied treadmill speed to achieve 45%-50% [34], while others varied speed to achieve 85% to 95% [35], [36] of one’s age-related maximum heart rate. Again, Pohl et al. have offered treadmill-assisted exercise to subjects with stroke while ensuring that the user’s heart rate settled to the respective resting-state heart rate [37]. Again of late, there had been advanced treadmills, available off-the-shelf, that can monitor one’s heart rate and vary the treadmill speed to maintain the user’s heart rate at a predefined level [38], [39]. Though one’s heart rate is an important indicator that needs to be considered during treadmill-assisted exercise, one’s walking speed while using the treadmill also offers important information on one’s exercise capability. This is because gait rehabilitation aims to improve one’s community ambulation that is related to one’s walking speed [40]. Thus, it would be interesting to explore the composite effect of one’s walking speed along with working and resting-state heart rates during treadmill-assisted gait exercise to study one’s energy expenditure, quantified in terms of a proxy index, namely Physiological Cost Index (PCI) [31].
Given that there are no existing studies that have used a treadmill-assisted gait exercise platform deciding the dosage of exercise intensity based on one’s PCI estimated in real-time during exercise, it might be interesting to explore the use of such an individualized gait exercise platform for individuals with stroke. Thus, we wanted to extend a treadmill-assisted gait exercise platform by making it adaptive to one’s individualized PCI. Additionally, we wanted to augment this platform with VR-based user interface to offer visual feedback to the user undergoing gait exercise. We hypothesized that such a gait exercise platform can recondition a patient’s exercise capability in terms of cardiac and gait performance to achieve improved community ambulation. The objectives of our research were three-fold, namely to (i) implement a novel PCI-sensitive Adaptive Response Technology (PCI-ART) offering VR-based treadmill-assisted gait exercise, (ii) investigate the safety and feasibility of use of this platform among able-bodied individuals before applying it to subjects with stroke and (iii) examine implications of undergoing gait exercise with this platform on the patients’ (a) cardiac and gait performance along with energy expenditure, (b) clinical measures estimating the physical reconditioning and (c) views on their community ambulation capabilities.
The rest of the paper is organized as follows: Section II presents our system design. Section III explains the experiments and procedures of this study. Section IV discusses the results. In Section V, we summarize our findings, limitations, and scope of future research.[…]
[ARTICLE] Portable Motion-Analysis Device for Upper-Limb Research, Assessment, and Rehabilitation in Non-Laboratory Settings – Full Text
Posted by Kostas Pantremenos in Paretic Hand, REHABILITATION on December 4, 2019
Abstract
SECTION I.
Introduction
An integral part of clinical care for individuals with motor disorders is to assess motor function to guide and evaluate medical treatment, surgical intervention or physical therapy. One of the challenges for assessing motor function is to define sensitive and quantitative measures that can be readily obtained in clinical practice. The objective of this study was to develop a device that affords quantitative assessment of motor impairments in non-laboratory settings. The specific focus is on individuals with upper-limb movement disorders. One central goal was to ground the task in scientific research to relate clinical measures to research and capitalize on insights from fundamental research.
This paper first lays out the need for such a device particularly for children with motor disorders and post-stroke rehabilitation. We then motivate the specific motor task that was originally conceived for basic research on motor control. We then detail the design of the prototype with all hardware and software components so that it can be replicated. One design goal was to make the device low-cost, so that it can be used in many clinical environments including at home for therapeutic exercises. We conclude with first results from pilot experiments acquired both in a traditional laboratory setting and in an Epilepsy Monitoring Unit. These first data were obtained from children with dystonia. However, the device is not limited to this population and is currently further modified for the assessment of stroke patients.
A. Clinical Assessments of Motor Disorders
A motor disorder manifests as an impaired ability to execute a movement with the intended spatial and temporal pattern. This includes abnormal posturing, presence of unintended excessive movement, and normal movements occurring at unintended or inappropriate times [1]. Patients with upper-limb impairments require special assistance to perform common motor tasks associated with self-care, such as feeding and dressing. Challenges in their movement control result in frustration, which leads to less engagement and practice, and thereby fewer opportunities to attenuate their motor disabilities and improve their movement control.
Motor disorder are observed also among children. Cerebral Palsy (CP) is a common cause of movement disorders among children, affecting 3 to 4 individuals per 1000 births in the US. The dyskinetic form of CP occurs in 15% of all cases [2]. Due to inflexible postures, caused by muscle spasms and contractures together with involuntary jerky movements, children with dyskinetic CP are often prevented from participation in many daily activities. This also prevents them from acquiring age-appropriate motor skills during critical periods of skill development [3], [4]. This is particularly aggravated when the condition affects the upper limbs.
For clinical motor assessments, the current standard tools are clinical scales. For cerebral palsy, typical tests are the gross motor function classification system (GMFCS) [5], the manual ability classification system (MACS) [6], the House Scale [7], the Melbourne Assessment [8], the Assisting Hand Assessment [9], the Hypertonia Assessment Tool (HAT) [10], the Barry-Albright Dystonia (BAD) scale [11], and the Shriners Hospital for Children Upper Extremity Evaluation [12]. These outcome measures were devised to satisfy the typical criteria for effective outcome measures, including reliability, validity, specificity, and responsiveness [13]. Although useful, these rating scales rely on subjective assessment and questionnaires that are vulnerable to inter-rater and test-retest reliability, nonlinearity, multi-dimensionality, and ceiling or floor effects [14]. These shortcomings need to be overcome by more quantitative outcome measures to provide a better evaluation of the individual’s motor functions and abilities, and potentially utilize such measures to objectvely assess and titrate interventions.
B. Quantitative Assessment of Motor Function
Motion tracking technologies have provided quantitative means of recording movements through a variety of sensing technology that tracks and stores movement. Camera-based motion capture, such as Vicon (Vicon Motion Systems, Oxford, UK) and Optitrak (Northern Digital Inc, Ontario, CA) requires external markers or sensors placed on key anatomical landmarks to reconstruct the skeletal model of human body parts. These state-of-the-art technologies track motion to very high precision with high sampling rates and they have been used for pre- and post-treatment assessment of upper- or lower-extremity pathologies. However, such data acquisition is limited to traditional laboratory settings because the multi-camera systems are expensive and not portable.
On the other hand, there are low-cost inertial measurement units (IMUs) that directly measure acceleration, rotational change and magnetic orientation. While these sensors have the advantage that they are self-contained and wearable, drawbacks are degraded accuracy due to drift, calibration errors and noise inherent to inertial sensors and the need to frequently recharge batteries for real-time data streaming [15]. Moreover, attaching sensors to body parts can be inconvenient or even impossible for certain clinical populations, and many children will not tolerate them.
In view of the above arguments, there is a strong need for less invasive devices that can provide quantitative measurements in tasks related to upper-extremeity motor function. Preferably, such a device should allow for portability and be low-cost to reach large populations.
C. Low-Cost Rehabilitation at Home
Rehabilitation follows standard practice and frequently requires one-on-one interaction with a therapist for extended periods of time. For these reasons, robotic devices have emerged to deliver higher-dosage and higher-intensity training for patients with movement disorders such as cerebral palsy and stroke [16]–[17][18][19]. However, while effective, robotic therapy is expensive and to date can only be used in clinical settings. To increase the volume in therapy, lower-cost devices that can be used at home are urgently needed.
Performance improvements with predominant home training are indeed possible. This was demonstrated by pediatric constraint-induced movement therapy (CIMT) for children with hemiparetic CP [20], [21]. Further, it was shown that even children with severe dystonia can improve their performance if they use an interface or device that enables and facilitates their severely handicapped movements [22].
A portable low-cost device for home use that is able to provide reliable quantitative measurements would help address the above shortcomings. Measurements could also be streamed to careproviders on a secure cloud protocol, for diagnosis of interventions, analysis of therapeutic outcomes, and further follow up.
D. Theoretically-Grounded Rehabilitation
Motor tasks for home therapy should be engaging to avoid boredom and attrition and should also have functional relevance. With this goal in mind, we developed a motor task that was motivated by the daily self-feeding activity of leading a cup of coffee or a spoon filled with soup to the mouth. The core challenge of actions of this kind is that moving such an object with sloshing liquid presents complex interaction forces: any force applied to the cup also applies a force to the liquid that then acts back on the hand. When such internal dynamics is present, interaction forces become quite complex, and the human performing the task needs to predict and preempt the internal dynamics of the moving liquid. Clearly, better understanding task is like guiding a cup of coffee to one’s mouth or a spoonful of soup has high functional relevance. While many such functional tasks have been developed for rehabiltation (e.g., the box-and-block and the pegboard task), the quantitative assessment should allow for more than descriptive outcome measures such as error or success rate. Monitoring the ‘process’ continuously should provide more detailed insight into coordinative challenges. This is indeed possible in the task of guiding a cup of coffee as we explain next.
In previous research, we abstracted a relevant, yet simplified model task, inspired by guiding a cup of coffee [23]–[24][25][26]. To reduce the complexity and afford theoretical analyses, the “cup of coffee” was simplified to a rigid object with a rolling ball inside. The rolling ball represents the moving liquid; this is also similar to the children’s game of transporting an egg in a spoon [27]. Fig.1A-C shows the transition from the real object to the simplified physical model. Importantly, the original task (Fig.1A) was reduced to a two-dimensional model, where the subject interacts with the object via a robotic manipulandum. The virtual model consists of a cart with a suspended pendulum, a well-known benchmark problem in control theory.
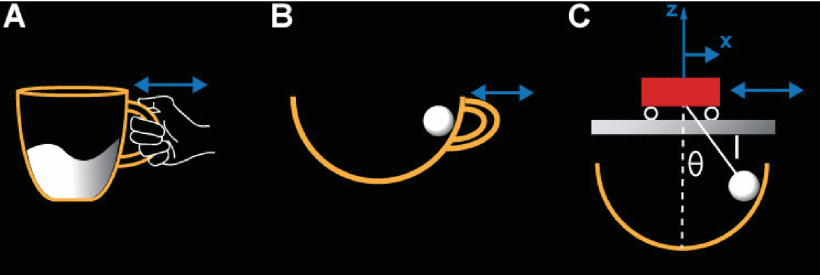
FIGURE 1.Model for the task of carrying a cup of coffee. A: The real object. B: The simplified physical model. C: The equivalent cart-and pendulum model implemented in the virtual task.
[…]
[Abstract] Kinematic analysis and control for upper limb robotic rehabilitation system – IEEE Conference Publication
Posted by Kostas Pantremenos in Paretic Hand, Rehabilitation robotics on July 7, 2018
Abstract
I. Introduction
Statistics shows that, at European Union level, the upper limb is second common body part injured, as a result of unintentional physical injury [1]. Also, one can note the shortage of therapists and high costs for patient or healthcare insurance systems. Current development in robotics may offer a solution for this problem [2], allowing the creation of robotic devices to support the rehabilitation process, in a supervised or unsupervised way, in physiotherapy clinics or at home. In this context, we proposed RAPMES, a new intelligent, adaptive robotic system, which can provide the rehabilitation protocols, defined by a therapist, for the wrist and elbow of upper limb, considering the patient reactions and based on real-time feedback. RAPMES robotic system is designed on an ongoing research project, which implies several stages of development. In a first stage, we conducted a study involving therapists, the personnel and devices existent in a physiotherapy clinic. The role of this study was to determine the requirements for the robotic device, and to reveal the specific therapeutic needs of patients with rehabilitation indications at wrist and elbow level. On a second stage, we used a real-time video motion analysis system, to determine and understand specific functional movements frequently made with the dominant upper limb, by healthy persons. One of our research objectives is to include these movements as a part of RAPMES control algorithm, as they may offer a better rehabilitation of the upper limb, for specific moves. Next, we designed the robotic device, based on findings described above, and realized an experimental model of the robotic device.
[Abstract] Development of a Minimal-Intervention-Based Admittance Control Strategy for Upper Extremity Rehabilitation Exoskeleton
Posted by Kostas Pantremenos in Rehabilitation robotics, Uncategorized on December 2, 2017
Abstract:
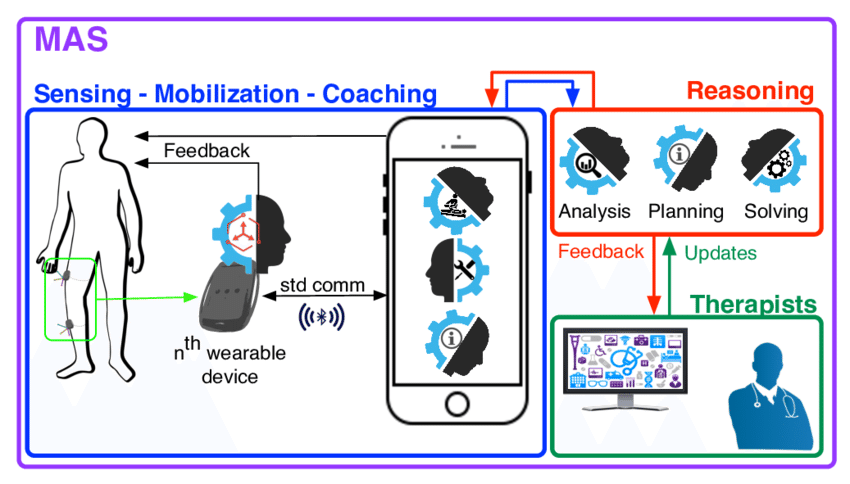
[ARTICLE] Agent-based systems for telerehabilitation: strengths, limitations and future challenges (PDF Download Available) – Full Text
Posted by Kostas Pantremenos in Tele/Home Rehabilitation on May 26, 2017